Abstract
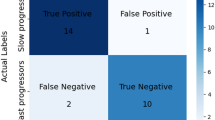
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders in the world. Previous studies have focused on the basal ganglia and cerebral cortices. To date, the cerebellum has not been systematically investigated in patients with PD. In the current study, 45 probable PD patients and 40 age- and gender-matched healthy controls underwent structural magnetic resonance imaging, and we used support vector machines combining with voxel-based morphometry to explore the cerebellar structural changes in the probable PD patients relative to healthy controls. The results revealed that the gray matter alterations were primarily located within the cerebellar Crus I, implying a possible important role of this region in PD. Furthermore, the gray matter alterations in the cerebellum could differentiate the probable PD patients from healthy controls with accuracies of more than 95 % (p < 0.001, permutation test) via cross-validation, suggesting the potential of analyzing the cerebellum in the clinical diagnosis of PD.


Similar content being viewed by others
References
Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. J Neurophysiol. 2013;7(2):193–224.
Shine JM, Matar E, Ward PB, Bolitho SJ, Gilat M, Pearson M, et al. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain. 2013;136:1204–15.
Sterling NW, Du G, Lewis MM, Dimaio C, Kong L, Eslinger PJ, et al. Striatal shape in Parkinson’ s disease. Neurobiol Aging. 2013;34:2510–6.
Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, et al. Missing pieces in the Parkinson’s disease puzzle. Nat Med. 2010;16:653–61.
Pyatigorskaya N, Gallea C, Garcia-Lorenzo D, Vidailhet M, Lehericy S. A review of the use of magnetic resonance imaging in Parkinson’s disease. Therapeut Adv Neurol Disord. 2014;7(4):12–26.
Melzer TR, Watts R, MacAskill MR, Pearson JF, Rueger S, Pitcher TL, et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson’s disease. Brain. 2011;134:845–55.
Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. NeuroImage. 2007;35:222–33.
Middleton FA, Strick PL. The cerebellum: an overview. Trends Cogn Sci. 1998;2(9):305–6.
Zhang K, Yu C, Zhang Y, Wu X, Zhu C, Chan P, et al. Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson’s disease. Eur J Radiol. 2011;77:269–73.
Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. NeuroImage. 2011;55:204–15.
Husárová I, Lungu OV, Mareček R, Mikl M, Gescheidt T, Krupa P, et al. Functional imaging of the cerebellum and basal ganglia during predictive motor timing in early Parkinson’s disease. J Neuroimaging. 2014;24(1):45–53.
Husárová I, Mikl M, Lungu OV, Mareček R, Vaníček J, Bareš M. Similar circuits but different connectivity patterns between the cerebellum, basal ganglia, and supplementary motor area in early Parkinson’s disease patients and controls during predictive motor timing. J Neuroimaging. 2013;23(4):452–62.
Joana B. Pereira CJ, Maria J. Martı’, Blanca Ramirez-Ruiz, David Bartre’s-Faz, Eduard Tolosa. Structural brain correlates of verbal fluency in Parkinson’s disease. NeuroReport. 2009;20(8):741-4.
Bar M. Visual objects in context. Nat Rev Neurosci. 2004;5(8):617–29.
Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29(26):8586–94.
Chaudhuri KR, Healy DG, Schapira AHV. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–45.
Baumann O, Mattingley JB. Functional topography of primary emotion processing in the human cerebellum. NeuroImage. 2012;61(4):805–11.
Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136:696–709.
Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113.
Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9.
Craddock RC, Holtzheimer PE, Hu XP, Mayberg HS. Disease state prediction from resting state functional connectivity. Magn Reson Med. 2009;62:1619–28.
Efron B, Tibshirani R. [Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy]: rejoinder. Statist Sci. 1986;1(1):77.
Zeng L-L, Shen H, Liu L, Wang L, Li B, Fang P, et al. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135:1498–507.
Zeng L-L, Liu L, Liu Y, Shen H, Li Y, Hu D. Antidepressant treatment normalizes white matter volume in patients with major depression. PLoS One. 2012;7(8):e44248.
Cherubini A, Nistico R, Novellino F, Salsone M, Nigro S, Donzuso G, et al. Magnetic resonance support vector machine discriminates essential tremor with rest tremor from tremor-dominant Parkinson disease. Mov Disord. 2014;29(9):1216–9.
Cherubini A, Morelli M, Rita N, Salsone M, Arabia G, Vasta R, et al. Magnetic resonance support vector machine discriminates between Parkinson Disease and progressive supranuclear palsy. Mov Disord. 2014;29(2):266–9.
Helmich RC, Bloem BR, Toni I. Motor imagery evokes increased somatosensory activity in Parkinson’s disease patients with tremor. Hum Brain Mapp. 2012;33:1763–79.
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–45.
Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–64.
Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42.
Zeng L-L, Wang D, Fox MD, Sabuncu M, Hu D, Ge M, et al. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci U S A. 2014;111(16):6058–62.
Rektorova I, Biundo R, Marecek R, Weis L, Aarsland D, Antonini A. Grey matter changes in cognitively impaired Parkinson’s disease patients. PLoS One. 2014;9(1):e85595.
Yarnall A, Archibald N, Burn D. Parkinson’s disease. Mov Disord. 2012;40(10):529–35.
Ekman U, Eriksson J, Forsgren L, Mo SJ, Riklund K, Nyberg L. Functional brain activity and presynaptic dopamine uptake in patients with Parkinson’s disease and mild cognitive impairment: a cross-sectional study. Lancet Neurol. 2012;11:679–87.
Ko JH, Mure H, Tang CC, Ma Y, Dhawan V, Spetsieris P, et al. Parkinson’s disease: increased motor network activity in the absence of movement. J Neurosci. 2013;33(10):4540–9.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–44.
Zeng L-L, Shen H, Liu L, Fang P, Liu Y, Hu D. State-dependent and trait-related gray matter changes in nonrefractory depression. NeuroReport. 2015;26:57–65.
Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107(18):8452–6.
Kitai ST, Kita H. The basal ganglia II—structure and function: current concepts. New York: Plenum; 1987.
Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006;5:75–86.
Aarts E, Nusselein AAM, Smittenaar P, Helmich RC, Bloem BR, Cools R. Greater striatal responses to medication in Parkinson’s disease are associated with better task-switching but worse reward performance. Neuropsychologia. 2014;62:390–7.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Xuanwu Hospital’s Medical Research Ethical Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This study was funded by the National Science Foundation of China (61503397, 61420106001, 61375111, and 61375034) and National High-tech Program of China (2012AA011601).
Rights and permissions
About this article
Cite this article
Zeng, LL., Xie, L., Shen, H. et al. Differentiating Patients with Parkinson’s Disease from Normal Controls Using Gray Matter in the Cerebellum. Cerebellum 16, 151–157 (2017). https://doi.org/10.1007/s12311-016-0781-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-016-0781-1