Abstract
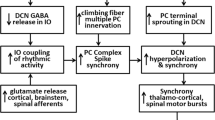
In this review, we hope to stimulate interest in animal models as opportunities to understand tremor mechanisms within the cerebellar system. We begin by considering the harmaline model of essential tremor (ET), which has ET-like anatomy and pharmacology. Harmaline induces the inferior olive (IO) to burst fire rhythmically, recruiting rhythmic activity in Purkinje cells (PCs) and deep cerebellar nuclei (DCN). This model has fostered the IO hypothesis of ET, which postulates that factors that promote excess IO, and hence PC complex spike synchrony, also promote tremor. In contrast, the PC hypothesis postulates that partial PC cell loss underlies tremor of ET. We describe models in which chronic partial PC loss is associated with tremor, such as the Weaver mouse, and others with PC loss that do not show tremor, such as the Purkinje cell degeneration mouse. We postulate that partial PC loss with tremor is associated with terminal axonal sprouting. We then discuss tremor that occurs with large lesions of the cerebellum in primates. This tremor has variable frequency and is an ataxic tremor not related to ET. Another tremor type that is not likely related to ET is tremor in mice with mutations that cause prolonged synaptic GABA action. This tremor is probably due to mistiming within cerebellar circuitry. In the final section, we catalog tremor models involving neurotransmitter and ion channel perturbations. Some appear to be related to the IO hypothesis of ET, while in others tremor may be ataxic or due to mistiming. In summary, we offer a tentative framework for classifying animal action tremor, such that various models may be considered potentially relevant to ET, subscribing to IO or PC hypotheses, or not likely relevant, as with mistiming or ataxic tremor. Considerable further research is needed to elucidate the mechanisms of tremor in animal models.

Similar content being viewed by others
References
Handforth A. Harmaline tremor: underlying mechanisms in a potential animal model of essential tremor. Tremor Other Hyperkinet Mov (N Y). 2012;2. pii: 02-92-769-1.
de Montigny C, Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res. 1973;53:81–95.
Llinás R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973;18:69–87.
Batini C, Buisseret-Delmas C, Conrath-Verrier M. Harmaline-induced tremor. I. Regional metabolic activity as revealed by [14C]2-deoxyglucose in cat. Exp Brain Res. 1981;42:371–82.
Stratton SE, Lorden JF. Effect of harmaline on cells of the inferior olive in the absence of tremor: differential response of genetically dystonic and harmaline-tolerant rats. Neuroscience. 1991;41:543–49.
Milner TE, Cadoret G, Lessard L, Smith AM. EMG analysis of harmaline-induced tremor in normal and three strains of mutant mice with Purkinje cell degeneration and the role of the inferior olive. J Neurophysiol. 1995;73:2568–77.
Llinás R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986;376:163–82.
Park YG, Park HY, Lee CJ, Choi S, Jo S, Choi H, et al. CaV3.1 is a tremor rhythm pacemaker in the inferior olive. Proc Natl Acad Sci U S A. 2010;107:10731–6.
Zhan X, Graf WM. Harmaline attenuates voltage--sensitive Ca2+ currents in neurons of the inferior olive. J Pharm Pharm Sci. 2012;15:657–68.
Schnitzler A, Münks C, Butz M, Timmermann L, Gross J. Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord. 2009;24:1629–35.
Dupuis MJ, Evrard FL, Jacquerye PG, Picard GR, Lermen OG. Disappearance of essential tremor after stroke. Mov Disord. 2010;25:2884–7.
Boecker H, Wills AJ, Ceballos-Baumann A, Samuel M, Thompson PD, Findley LJ, et al. The effect of ethanol on alcohol-responsive essential tremor: a positron emission tomography study. Ann Neurol. 1996;39:650–58.
Hallett M, Dubinsky RM. Glucose metabolism in the brain of patients with essential tremor. J Neurol Sci. 1993;114:45–8.
McMahon A, Fowler SC, Perney TM, Akemann W, Knöpfel T, Joho RH. Allele-dependent changes of olivocerebellar circuit properties in the absence of the voltage-gated potassium channels Kv3.1 and Kv3.3. Eur J Neurosci. 2004;19:3317–27.
Hurlock EC, McMahon A, Joho RH. Purkinje-cell-restricted restoration of Kv3.3 function restores complex spikes and rescues motor coordination in Kcnc3 mutants. J Neurosci. 2008;28:4640–8.
Lang EJ, Tang T, Suh CY, Xiao J, Kotsurovskyy Y, Blenkinsop TA, et al. Modulation of Purkinje cell complex spike waveform by synchrony levels in the olivocerebellar system. Front Syst Neurosci. 2014;8:210.
Miwa H, Nishi K, Fuwa T, Mizuno Y. Differential expression of c-Fos following administration of two tremorgenic agents: harmaline and oxotremorine. Neuroreport. 2000;11:2385–90.
Oldenbeuving AW, Eisenman LM, De Zeeuw CI, Ruigrok TJ. Inferior olivary-induced expression of Fos-like immunoreactivity in the cerebellar nuclei of wild-type and Lurcher mice. Eur J Neurosci. 1999;11:3809–22.
Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008;14:75–80.
Lang EJ. Excitatory afferent modulation of complex spike synchrony. Cerebellum. 2003;2:165–70.
Lang EJ. GABAergic and glutamatergic modulation of spontaneous and motor-cortex-evoked complex spike activity. J Neurophysiol. 2002;87:1993–2008.
Lang EJ, Sugihara I, Llinás R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76:255–75.
Lefler Y, Yarom Y, Uusisaari MY. Cerebellar inhibitory input to the inferior olive decreases electrical coupling and blocks subthreshold oscillations. Neuron. 2014;81:1389–400.
Mignani S, Bohme GA, Birraux G, Boireau A, Jimonet P, Damour D, et al. 9-Carboxymethyl-5H,10H-imidazo[1,2-a]indeno[1,2-e]pyrazin-4-one-2-carbocylic acid (RPR117824): selective anticonvulsive and neuroprotective AMPA antagonist. Bioorg Med Chem. 2002;10:1627–37.
Paterson NE, Malekiani SA, Foreman MM, Olivier B, Hanania T. Pharmacological characterization of harmaline-induced tremor activity in mice. Eur J Pharmacol. 2009;616:73–80.
Shaffer CL, Hurst RS, Scialis RJ, Osgood SM, Bryce DK, Hoffmann WE, et al. Positive allosteric modulation of AMPA receptors from efficacy to toxicity: the interspecies exposure-response continuum of the novel potentiator PF-4778574. J Pharmacol Exp Ther. 2013;347:212–24.
Ondo WG, Jankovic J, Connor GS, Pahwa R, Elble R, Stacy MA, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66:672–7.
Handforth A, Homanics GE, Covey DF, Krishnan K, Lee JY, Sakimura K, et al. T-type calcium channel antagonists suppress tremor in two mouse models of essential tremor. Neuropharmacology. 2010;59:380–7.
Handforth A, Martin FC, Kang GA, Vanek Z. Zonisamide for essential tremor: an evaluator-blinded study. Mov Disord. 2009;24:437–40.
Blenkinsop TA, Lang EJ. Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci. 2006;26:1739–48.
Martin FC, Handforth A. Carbenoxolone and mefloquine suppress tremor in the harmaline mouse model of essential tremor. Mov Disord. 2006;21:1641–9.
McCormick DA, Steinmetz JE, Thompson RF. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Res. 1985;359:120–30.
Kronenbuerger M, Tronnier VM, Gerwig M, Fromm C, Coenen VA, Reinacher P, et al. Thalamic deep brain stimulation improves eyeblink conditioning deficits in essential tremor. Exp Neurol. 2008;211:387–96.
Marshall SP, Lang EJ. Local changes in the excitability of the cerebellar cortex produce spatially restricted changes in complex spike synchrony. J Neurosci. 2009;29:14352–62.
Handforth A. Use of the harmaline and α1 knockout models to Identify molecular targets for essential tremor. In: LeDoux MS, editor. Movement disorders: genetics and models. 2nd ed. San Diego: Elsevier, Academic; 2015. p. 615–29.
Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor. Parkinsonism Relat Disord. 2011;17:406–09.
Kayakabe M, Kakizaki T, Kaneko R, Sasaki A, Nakazato Y, Shibasaki K, et al. Motor dysfunction in cerebellar Purkinje cell-specific vesicular GABA transporter knockout mice. Front Cell Neurosci. 2014;7:286.
Riedel CJ, Muraszko KM, Youle RJ. Diphtheria toxin mutant selectively kills cerebellar Purkinje neurons. Proc Natl Acad Sci U S A. 1990;87:5051–55.
Angner RT, Kelly RM, Wiley RG, Walsh TJ, Reuhl KR. Preferential destruction of cerebellar Purkinje cells by OX7-saporin. Neurotoxicology. 2000;21:395–403.
Shamir M, Perl S, Sharon L. Late onset of cerebellar abiotrophy in a Siamese cat. J Small Anim Pract. 1999;40:343–45.
Jacquelin C, Strazielle C, Lalonde R. Neurologic function during developmental and adult stages in Dab1 scm (scrambler) mutant mice. Behav Brain Res. 2012;226:265–73.
Sarna JR, Hawkes R. Patterned Purkinje cell loss in the ataxic sticky mouse. Eur J Neurosci. 2011;34:79–86.
Bäurle J, Hoshi M, Grüsser-Cornehls U. Dependence of parvalbumin expression on Purkinje cell input in the deep cerebellar nuclei. J Comp Neurol. 1998;392:499–514.
Lalonde R, Strazielle C. Spontaneous and induced mouse mutations with cerebellar dysfunctions: behavior and neurochemistry. Brain Res. 2007;1140:51–74.
Traka M, Millen KJ, Collins D, Elbaz B, Kidd GJ, Gomez CM, et al. WDR81 is necessary for purkinje and photoreceptor cell survival. J Neurosci. 2013;33:6834–44.
Duchala CS, Shick HE, Garcia J, Deweese DM, Sun X, Stewart VJ, et al. The toppler mouse: a novel mutant exhibiting loss of Purkinje cells. J Comp Neurol. 2004;16(476):113–29.
Perkins EM, Clarkson YL, Sabatier N, Longhurst DM, Millward CP, Jack J, et al. Loss of β-III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of SCA5 in humans. J Neurosci. 2010;30:4857–67.
Clark BR, LaRegina M, Tolbert DL. X-linked transmission of the shaker mutation in rats with hereditary Purkinje cell degeneration and ataxia. Brain Res. 2000;858:264–73.
Anderson L, Rossi D, Linehan J, Brandner S, Weissmann C. Transgene-driven expression of the Doppel protein in Purkinje cells causes Purkinje cell degeneration and motor impairment. Proc Natl Acad Sci U S A. 2004;101:3644–9.
Grüsser-Cornehls U, Grüsser C, Bäurle J. Vermectomy enhances parvalbumin expression and improves motor performance in Weaver mutant mice: an animal model for cerebellar ataxia. Neuroscience. 1999;91:315–26.
Roffler-Tarlov S, Beart PM, O'Gorman S, Sidman RL. Neurochemical and morphological consequences of axon terminal degeneration in cerebellar deep nuclei of mice with inherited Purkinje cell degeneration. Brain Res. 1979;168:75–95.
Shojaeian H, Delhaye-Bouchaud N, Mariani J. Stability of inferior olivary neurons in rodents. I. Moderate cell loss in adult Purkinje cell degeneration mutant mouse. Brain Res. 1988;466:211–8.
Bäurle J, Grüsser-Cornehls U. Differential number of glycine- and GABA-immunopositive neurons and terminals in the deep cerebellar nuclei of normal and Purkinje cell degeneration mutant mice. J Comp Neurol. 1997;382:443–58.
Bäurle J, Grover BG, Grüsser-Cornehls U. Plasticity of GABAergic terminals in Deiters’ nucleus of weaver mutant and normal mice: a quantitative light microscopic study. Brain Res. 1992;591:305–18.
Roffler-Tarlov S, Turey M. The content of amino acids in the developing cerebellar cortex and deep cerebellar nuclei of granule cell deficient mutant mice. Brain Res. 1982;247:65–73.
Heckroth JA, Abbott LC. Purkinje cell loss from alternating sagittal zones in the cerebellum of leaner mutant mice. Brain Res. 1994;658(1-2):93–104.
Grüsser-Cornehls U, Luy M, Bäurle J. Electrophysiology and GABA-immunocytochemistry in the vestibular nuclei of normal (C57BL/6J) and Leaner mutant mice. Brain Res. 1995;703:51–62.
Zanjani H, Herrup K, Mariani J. Cell number in the inferior olive of nervous and leaner mutant mice. J Neurogenet. 2004;18:327–39.
Frederic F, Hainaut F, Thomasset M, Guenet JL, Delhaye-Bouchaud N, Mariani J. Cell counts of Purkinje and inferior olivary neurons in the ‘hyperspiny Purkinje cells’ mutant mouse. Eur J Neurosci. 1992;4:127–35.
Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS, et al. SCA1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell. 1995;82:937–48.
Huynh DP, Figueroa K, Hoang N, Pulst SM. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet. 2000;26:44–50.
Nandy K. Morphological changes in the cerebellar cortex of aging Macaca nemestrina. Neurobiol Aging. 1981;2:61–4.
Sturrock RR. Age related changes in Purkinje cell number in the cerebellar nodulus of the mouse. J Hirnforsch. 1989;30:757–60.
Babij R, Lee M, Cortés E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136(Pt 10):3051–61.
Carpenter MB, Stevens GH. Structural and functional relationships between the deep cerebella; nuclei and the brachium conjunctivum in the rhesus monkey. J Comp Neurol. 1957;107:109–63.
Goldberger ME, Growden JH. Tremor at rest following cerebellar lesions in monkeys: effect of L-DOPA administration. Brain Res. 1971;27:183–7.
Vilis T, Hore J. Effects of changes in mechanical state of limb on cerebellar intention tremor. J Neurophysiol. 1977;40:1214–24.
Gemba H, Sasaki K, Yoneda Y, Hashimoto S, Mizuno N. Tremor in the monkey with a cerebellar lesion. Exp Neurol. 1980;69:173–82.
Nixon PD, Passingham RE. The cerebellum and cognition: cerebellar lesions do not impair spatial working memory or visual associative learning in monkeys. Eur J Neurosci. 1999;11:4070–80.
Aring CD, Fulton JF. Relation of the cerebrum to the cerebellum. Arch Newot Psychiat. 1936;35:439–66.
Ferguson SA. Neuroanatomical and functional alterations resulting from early postnatal cerebellar insults in rodents. Pharmacol Biochem Behav. 1996;55:663–71.
Anderson WJ, Stromberg MW. Effects of low-level x-irradiation on cat cerebella at different postnatal intervals. I. Quantitative evaluation of morphological changes. J Comp Neurol. 1977;171:17–37.
Duffell S, Lock EA. Re-evaluation of archival material for neuronal cell injury produced by L-2-chloropropionic acid in the rat brain. Neurotoxicology. 2004;25:1031–40.
Flegel T, Matiasek K, Henke D, Grevel V. Cerebellar cortical degeneration with selective granule cell loss in Bavarian mountain dogs. J Small Anim Pract. 2007;48:462–65.
Wagner SO, Podell M, Fenner WR. Generalized tremors in dogs: 24 cases (1984-1995). J Am Vet Med Assoc. 1997;211:731–5.
Homanics GE, Elsen FP, Ying SW, Jenkins A, Ferguson C, Sloat B, et al. A gain-of-function mutation in the GABA receptor produces synaptic and behavioral abnormalities in the mouse. Genes Brain Behav. 2005;4:10–9.
Chiu CS, Brickley S, Jensen K, Southwell A, Mckinney S, Cull-Candy S, et al. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci. 2005;25:3234–45.
Gironell A, Martínez-Corral M, Pagonabarraga X, Kulisevsky J. Tiagabine for essential tremor: an open-label trial. Mov Disord. 2008;23:1955–56.
Ogris W, Lehner R, Fuchs K, Furtmüller B, Höger H, Homanics GE, et al. Investigation of the abundance and subunit composition of GABAA receptor subtypes in the cerebellum of α1-subunit-deficient mice. J Neurochem. 2006;96:136–47.
Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of γ-aminobutyric acid type A receptor α1 subunit knockout mice. J Comp Neurol. 2006;495:408–21.
Kralic JE, Criswell HE, Osterman JL, O'Buckley TK, Wilkie ME, Matthews DB, et al. Genetic essential tremor in γ-aminobutyric acidA receptor α1 subunit knockout mice. J Clin Invest. 2005;115:774–9.
Deng H, Xie WJ, Le WD, Huang MS, Jankovic J. Genetic analysis of the GABRA1 gene in patients with essential tremor. Neurosci Lett. 2006;401:16–9.
Ortinski PI, Turner JR, Barberis A, Motamedi G, Yasuda RP, Wolfe BB, et al. Deletion of the GABAA receptor α1 subunit increases tonic GABAA receptor current: a role for GABA uptake transporters. J Neurosci. 2006;26:9323–31.
Uusisaari M, Knöpfel T. Functional classification of neurons in the mouse lateral cerebellar nuclei. Cerebellum. 2011;10:637–46.
Sillevis Smitt P, Kinoshita A, De Leeuw B, Moll W, Coesmans M, Jaarsma D, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000;342:21–7.
Conti V, Aghaie A, Cilli M, Martin N, Caridi G, Musante L, et al. crv4, a mouse model for human ataxia associated with kyphoscoliosis caused by an mRNA splicing mutation of the metabotropic glutamate receptor 1 (Grm1). Int J Mol Med. 2006;18:593–600.
Rossi PI, Musante I, Summa M, Pittaluga A, Emionite L, Ikehata M, et al. Compensatory molecular and functional mechanisms in nervous system of the Grm1 crv4 mouse lacking the mGlu1 receptor: a model for motor coordination deficits. Cereb Cortex. 2013;23:2179–89.
Kolasiewicz W, Kuter K, Wardas J, Ossowska K. Role of the metabotropic glutamate receptor subtype 1 in the harmaline-induced tremor in rats. J Neural Transm. 2009;116:1059–63.
Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007;6:389–400.
Sugihara I, Lang EJ, Llinás R. Serotonin modulation of inferior olivary oscillations and synchronicity: a multiple-electrode study in the rat cerebellum. Eur J Neurosci. 1995;7:521–34.
Becker L, von Wegerer J, Schenkel J, Zeilhofer HU, Swandulla D, Weiher H. Disease-specific human glycine receptor α1 subunit causes hyperekplexia phenotype and impaired glycine- and GABAA-receptor transmission in transgenic mice. J Neurosci. 2002;22:2505–12.
Knaus HG, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LM, et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33:5819–28.
Imlach WL, Finch SC, Dunlop J, Meredith AL, Aldrich RW, Dalziel JE. The molecular mechanism of “ryegrass staggers,” a neurological disorder of K+ channels. J Pharmacol Exp Ther. 2008;327:657–64.
Cavanagh JB, Holton JL, Nolan CC, Ray DE, Naik JT, Mantle PG. The effects of the tremorgenic mycotoxin penitrem A on the rat cerebellum. Vet Pathol. 1998;35:53–63.
Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101:9474–8.
Chen X, Kovalchuk Y, Adelsberger H, Henning HA, Sausbier M, Wietzorrek G, et al. Disruption of the olivo-cerebellar circuit by Purkinje neuron-specific ablation of BK channels. Proc Natl Acad Sci U S A. 2010;107:12323–8.
Szatanik M, Vibert N, Vassias I, Guénet JL, Eugène D, de Waele C, et al. Behavioral effects of a deletion in Kcnn2, the gene encoding the SK2 subunit of small-conductance Ca2+-activated K+ channels. Neurogenetics. 2008;9:237–48.
O’Brien JE, Meisler MH. Sodium channel SCN8A (Nav1.6): properties and de novo mutations in epileptic encephalopathy and intellectual disability. Front Genet. 2013;4:213.
Levin SI, Khaliq ZM, Aman TK, Grieco TM, Kearney JA, Raman IM, et al. Impaired motor function in mice with cell-specific knockout of sodium channel Scn8a (NaV1.6) in cerebellar purkinje neurons and granule cells. J Neurophysiol. 2006;96:785–93.
Sharkey LM, Jones JM, Hedera P, Meisler MH. Evaluation of SCN8A as a candidate gene for autosomal dominant essential tremor. Parkinsonism Relat Disord. 2009;15:321–23.
Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from NaV1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci. 2007;27:11065–74.
Ueno T, Kameyama K, Hirata M, Ogawa M, Hatsuse H, Takagaki Y, et al. A mouse with a point mutation in plasma membrane Ca2+-ATPase isoform 2 gene showed the reduced Ca2+ influx in cerebellar neurons. Neurosci Res. 2002;42:287–97.
Kodama T, Itsukaichi-Nishida Y, Fukazawa Y, Wakamori M, Miyata M, Molnar E, et al. A CaV2.1 calcium channel mutation rocker reduces the number of postsynaptic AMPA receptors in parallel fiber-Purkinje cell synapses. Eur J Neurosci. 2006;24:2993–3007.
Rhyu IJ, Oda S, Uhm CS, Kim H, Suh YS, Abbott LC. Morphologic investigation of rolling mouse Nagoya (tg rol /tg rol) cerebellar Purkinje cells: an ataxic mutant, revisited. Neurosci Lett. 1999;266:49–52.
Plomp JJ, van den Maagdenberg AM, Kaja S. The ataxic Cacna1a-mutant mouse rolling Nagoya: an overview of neuromorphological and electrophysiological findings. Cerebellum. 2009;8:222–30.
Meier H, MacPike AD. Three syndromes produced by two mutant genes in the mouse. Clinical, pathological, and ultrastructural bases of tottering, leaner, and heterozygous mice. J Hered. 1971;62:297–302.
Acknowledgments
We are grateful to Nicholas Franich, Ph.D. for the assistance with figure. The author was supported by Veterans Affairs. The author has received clinical research funding from Cyberonics Inc., Medtronic, UCB Pharma, Ortho-McNeil, Forest Laboratories, Sonexa, and Eisai.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no conflict of interest either directly or indirectly related to this research.
Rights and permissions
About this article
Cite this article
Handforth, A. Linking Essential Tremor to the Cerebellum—Animal Model Evidence. Cerebellum 15, 285–298 (2016). https://doi.org/10.1007/s12311-015-0750-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0750-0