Abstract
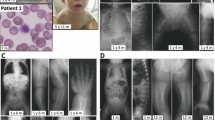
Phosphomannomutase 2 deficiency (PMM2-CDG) patients may present as mild phenotypes, with the cerebellum frequently involved. In those cases, false-negative results in screening may occur when applying conventional biochemical procedures. Our aim was to report two patients with a diagnosis of PMM2-CDG presenting with mild clinical phenotype. Patient 1—at 9 months of age, she presented with just psychomotor delay, tremor, hypotonia, and slight lipodystrophy. Patient 2—she presented at 8 months of age with psychomotor delay, hand stereotypes, hypotonia, convergent bilateral strabismus, and tremor but no lipodystrophy. Routine biochemical parameters including blood count, clotting factors, proteins, and thyroid hormone were normal in both cases. Cranial MRI evidenced mild cerebellar atrophy with moderate vermis hypoplasia. In case 1, sialotransferrin pattern showed very slightly increased disialotransferrin with no asialotransferrin, and in case 2, the transferrin pattern was impaired in the first study but nearly normal in the second. Nevertheless, in all the samples, quantification of the patterns obtained by capillary zone electrophoresis analysis gave results out of the control range. High residual PMM2 activity was observed in both cases and the genetic analysis showed that patient 1 was heterozygous for c.722G>C (p.C241S) and c.368G>A (p.R123Q) mutations, and patient 2 showed the c.722G>C and the c.470T>C (p.F157S) mutations in the PMM2 gene. We would like to stress the importance of the use of sensitive semiquantitative methods of screening for CDG in order to achieve early identification of patients with mild phenotypes. Intentional tremor was an atypical but remarkable clinical feature in both cases, and the global cerebellar atrophy with vermis hypoplasia reinforced the early clinical suspicion of a PMM2-CDG disease.


Similar content being viewed by others
References
Jaeken J, Vanderschueren-Lodeweyckx M, Casaer P, Snoeck L, Corbeel L, Eggermont E, et al. Familial psychomotor retardation with markedly fluctuating serum prolactin, FSH and GH levels, partial TBG-deficiency, increased serum arylsulfatase-A and increased CSF protein—new syndrome? Pediatr Res. 1980;14:179.
Marquardt T, Denecke J. Congenital disorders of glycosylation: review of their molecular bases, clinical presentations and specific therapies. Eur J Pediatr. 2003;162:359–79.
Imtiaz F, Worthington V, Champion M, Beesley C, Charlwood J, Clayton P, et al. Genotypes and phenotypes of patients in the UK with carbohydrate-deficient glycoprotein syndrome type 1. J Inherit Metab Dis. 2000;23:162–74.
Enns GM, Steiner RD, Buist N, Cowan C, Leppig KA, McCracken MF, et al. Clinical and molecular features of congenital disorder of glycosylation in patients with type 1 sialotransferrin pattern and diverse ethnic origins. J Pediatr. 2002;141:695–700.
Briones P, Vilaseca MA, Schollen E, Ferrer I, Maties M, Busquets C, et al. Biochemical and molecular studies in 26 Spanish patients with congenital disorder of glycosylation type Ia. J Inherit Metab Dis. 2002;25:635–46.
de Lonlay P, Seta N, Barrot S, Chabrol B, Drouin V, Gabriel BM, et al. A broad spectrum of clinical presentation in congenital disorders of glycosylation I: a series of 26 cases. J Med Genet. 2001;38:14–9.
Van Schaftingen E, Jaeken J. Phosphomannomutase deficiency is a cause of carbohydrate-deficient glycoprotein syndrome type I. FEBS Lett. 1995;377:318–20.
Van de Kamp JM, Lefeber DJ, Ruijter GJ, Steggerda SJ, Den Hollander NS, Willems SM, et al. Congenital disorder of glycosylation type Ia presenting with hydrops fetalis. J Med Genet. 2007;44:277–80.
Truin G, Guillard M, Lefeber DJ, Sykut-Cegielska J, Adamowicz M, Hoppenreijs E, et al. Pericardial and abdominal fluid accumulation in congenital disorder of glycosylation type Ia. Mol Genet Metab. 2008;94:481–4.
Grünewald S. The clinical spectrum of phosphomannomutase 2 deficiency (CDG-Ia). Biochim Biophys Acta. 2009;1792:827–34.
Pancho C, Garcia-Cazorla A, Varea V, Artuch R, Ferrer I, Vilaseca MA, et al. Congenital disorder of glycosylation type Ia revealed by hypertransaminasemia and failure to thrive in a young boy with normal neurodevelopment. J Pediatr Gastroenterol Nutr. 2005;40:230–2.
Vermeer S, Kremer HPH, Leijten QH, Scheffer H, Matthijs G, Wevers RA, et al. Cerebellar ataxia and congenital disorder of glycosylation Ia (CDG-Ia) with normal routine CDG screening. J Neurol. 2007;254:1356–8.
Miossec-Chauvet E, Mikaeloff Y, Heron D, Merzoug V, Cormier-Daire V, de Lonlay P, et al. Neurological presentation in pediatric patients with congenital disorders of glycosylation type Ia. Neuropediatrics. 2003;34:1–6.
Boddaert N, Desguerre I, Bahi-Buisson N, Romano S, Valayannopoulos V, Saillour Y, et al. Posterior fossa imaging in 158 children with ataxia. J Neuroradiol. 2010;37:220–30.
Morava E, Cser B, Kárteszi J, Huijben K, Szonyi L, Kosztolanyi G, et al. Screening for CDG type Ia in Joubert syndrome. Med Sci Monit. 2004;10:CR469–72.
Drouin-Garraud V, Belgrand M, Grünewald S, Seta N, Dacher JN, Hénocq A, et al. Neurological presentation of a congenital disorder of glycosylation CDG-Ia: implications for diagnosis and genetic counseling. Am J Med Genet. 2001;101:46–9.
Pérez B, Briones P, Quelhas D, Artuch R, Vega AI, Quintana E, et al. The molecular landscape of phosphomannose mutase deficiency in Iberian Peninsula: Identification of fifteen population-specific mutations. J Inher Metab Dis, in press.
Vega AI, Pérez-Cerda C, Abia D, Gámez A, Briones P, Artuch R, et al. Expression analysis revealing destabilizing mutations in phosphomannomutase 2 deficiency--congenital disorder of glycosylation. J Inher Metab Dis, in press.
Quintana E, Montero R, Casado M, Navarro-Sastre A, Vilaseca MA, Briones P, et al. Comparison between high performance liquid chromatography and capillary zone electrophoresis for the diagnosis of congenital disorders of glycosylation. J Chromatogr B. 2009;877:2513–8.
Carchon HA, Chevigné R, Falmagne JB, Jaeken J. Diagnosis of congenital disorders of glycosylation by capillary zone electrophoresis of serum transferrin. Clin Chem. 2004;50:101–11.
Van Eijk HG, Van Noort WL, Dubelaar ML, Van der Heul C. The microheterogeneity of human transferrins in biological fluids. Clin Chim Acta. 1983;132:167–71.
Mader I, Döbler-Neumann M, Küker W, Stibler H, Krägeloh-Mann I. Congenital disorder of glycosylation type Ia: benign clinical course in a new genetic variant. Child’s Nerv Syst. 2002;18:77–80.
Kjaergaard S, Schwartz M, Skovby F. Congenital disorder of glycosylation type Ia (CDG-Ia): phenotypic spectrum of the R141H/F119L genotype. Arch Dis Child. 2001;85:236–9.
Dupre T, Cuer M, Barrot S, Barnier A, Cormier-Daire V, Munnich A, et al. Congenital disorder of glycosylation Ia with deficient phosphomannomutase activity but normal plasma glycoprotein pattern. Clin Chem. 2001;47:132–4.
Fletcher JM, Matthijs G, Jaeken J, Van Schaftingen E, Nelson PV. Carbohydrate-deficient glycoprotein syndrome: beyond the screen. J Inherit Metab Dis. 2000;23:396–8.
Hanh SH, Minnich SJ, O’Brien JF. Stabilization of hypoglycosylation in a patient with congenital disorder of glycosylation type Ia. J Inherit Metab Dis. 2006;29:235–7.
Stibler H, Blennow G, Kristiansson B, Lindehammer H, Hagberg B. Carbohydrate deficient glycoprotein syndrome: clinical expression in adults with a new metabolic disease. J Neurol Neurosurg Psychiatry. 1994;57:552–6.
Westphal V, Peterson S, Patterson M, Tournay A, Blumenthal A, Treacy EP, et al. Functional significance of PMM2 mutations in mildly affected patients with congenital disorders of glycosylation Ia. Genet Med. 2001;3:393–8.
Acknowledgments
This work was supported by grants from the Fondo de Investigación Sanitaria (FIS PI080663, PI080307, and PI10/00455). The CIBERER is an initiative of the Instituto de Salud Carlos III (ISCIII, MICIN, Spain). R.Artuch and M. Casado are supported by the program Intensificacion de la Actividad Investigadora (ISCIII). R Montero and MM O’Callaghan were funded by the CIBERER.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casado, M., O’Callaghan, M.M., Montero, R. et al. Mild Clinical and Biochemical Phenotype in Two Patients with PMM2-CDG (Congenital Disorder of Glycosylation Ia). Cerebellum 11, 557–563 (2012). https://doi.org/10.1007/s12311-011-0313-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-011-0313-y