Abstract
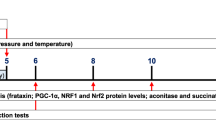
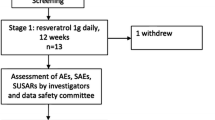
Friedreich ataxia (FRDA) is an autosomal recessive inherited neurodegenerative disorder leading to reduced expression of the mitochondrial protein frataxin. Previous studies showed frataxin upregulation in FRDA following treatment with recombinant human erythropoietin (rhuEPO). Dose–response interactions between frataxin and rhuEPO have not been studied until to date. We administered escalating rhuEPO single doses (5,000, 10,000 and 30,000 IU) in monthly intervals to five adult FRDA patients. Measurements of frataxin, serum erythropoietin levels, iron metabolism and mitochondrial function were carried out. Clinical outcome was assessed using the “Scale for the assessment and rating of ataxia”. We found maximal erythropoietin serum concentrations 24 h after rhuEPO application which is comparable to healthy subjects. Frataxin levels increased significantly over 3 months, while ataxia rating did not reveal clinical improvement. All FRDA patients had considerable ferritin decrease. NADH/NAD ratio, an indicator of mitochondrial function, increased following rhuEPO treatment. In addition to frataxin upregulation in response to continuous low-dose rhuEPO application shown in previous studies, our results indicate for a long-lasting frataxin increase after single high-dose rhuEPO administration. To detect frataxin-derived neuroprotective effects resulting in clinically relevant improvement, well-designed studies with extended time frame are required.




Similar content being viewed by others
References
Harding AE. Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 1981;104:589–620.
Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med. 2004;82:510–29.
Delatycki MB, Paris DB, Gardner RJ, Nicholson GA, Nassif N, Storey E, et al. Clinical and genetic study of Friedreich ataxia in an Australian population. Am J Med Genet. 1999;87:168–74.
Milbrandt TA, Kunes JR, Karol LA. Friedreich’s ataxia and scoliosis: the experience at two institutions. J Pediatr Orthop. 2008;28:234–8.
Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, et al. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–7.
Pianese L, Turano M, Lo Casale MS, De Biase I, Giacchetti M, Monticelli A, et al. Real time PCR quantification of frataxin mRNA in the peripheral blood leucocytes of Friedreich ataxia patients and carriers. J Neurol Neurosurg Psychiatry. 2004;75:1061–3.
Rouault TA, Tong WH. Iron-sulfur cluster biogenesis and human disease. Trends Genet. 2008;24:398–407.
Rotig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, et al. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet. 1997;17:215–7.
Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–74.
Siren AL, Fasshauer T, Bartels C, Ehrenreich H. Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system. Neurotherapeutics. 2009;6:108–27.
Sturm B, Stupphann D, Kaun C, Boesch S, Schranzhofer M, Wojta J, et al. Recombinant human erythropoietin: effects on frataxin expression in vitro. Eur J Clin Invest. 2005;35:711–7.
Boesch S, Sturm B, Hering S, Goldenberg H, Poewe W, Scheiber-Mojdehkar B. Friedreich’s ataxia: clinical pilot trial with recombinant human erythropoietin. Ann Neurol. 2007;62:521–4.
Boesch S, Sturm B, Hering S, Scheiber-Mojdehkar B, Steinkellner H, Goldenberg H, et al. Neurological effects of recombinant human erythropoietin in Friedreich’s ataxia: a clinical pilot trial. Mov Disord. 2008;23:1940–4.
Burk K, Malzig U, Wolf S, Heck S, Dimitriadis K, Schmitz-Hubsch T, et al. Comparison of three clinical rating scales in Friedreich ataxia (FRDA). Mov Disord. 2009;24:1779–84.
Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–20.
Steinkellner H, Scheiber-Mojdehkar B, Goldenberg H, Sturm B. A high throughput electrochemiluminescence assay for the quantification of frataxin protein levels. Anal Chim Acta. 2010;659:129–32.
Cox C, Camus P, Duvivier J. An enzymatic cycling procedure for NAD+ using an irreversible reaction with NAD+-peroxidase. Anal Biochem. 1982;119:185–93.
Oexle H, Gnaiger E, Weiss G. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim Biophys Acta. 1999;1413:99–107.
Erslev AJ. Erythropoietin titers in health and disease. Semin Hematol. 1991;28:2–7. discussion 7–8.
Heatherington AC. Clinical pharmacokinetic properties of rhuEPO: a review. In: Molineux G, Foot MA, Elliott SG, editors. Erythropoietins and erythropoiesis molecular, cellular, preclinical, and clinical biology. Boston: Birkhäuser Basel; 2003. p. 87–112.
Ramakrishnan R, Cheung WK, Wacholtz MC, Minton N, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after single and multiple doses in healthy volunteers. J Clin Pharmacol. 2004;44:991–1002.
Sacca F, Piro R, De Michele G, Acquaviva F, Antenora A, Carlomagno G, et al. Epoetin alfa increases frataxin production in Friedreich’s ataxia without affecting hematocrit. Mov Disord. 2010;26:739–42.
Means Jr RT. Recent developments in the anemia of chronic disease. Curr Hematol Rep. 2003;2:116–21.
Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23.
Busfield SJ, Tilbrook PA, Callus BA, Spadaccini A, Kuhn L, Klinken SP. Complex regulation of transferrin receptors during erythropoietin-induced differentiation of J2E erythroid cells—elevated transcription and mRNA stabilisation produce only a modest rise in protein content. Eur J Biochem. 1997;249:77–84.
Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007;78:183–205.
Weiss G, Houston T, Kastner S, Johrer K, Grunewald K, Brock JH. Regulation of cellular iron metabolism by erythropoietin: activation of iron-regulatory protein and upregulation of transferrin receptor expression in erythroid cells. Blood. 1997;89:680–7.
Campanella A, Rovelli E, Santambrogio P, Cozzi A, Taroni F, Levi S. Mitochondrial ferritin limits oxidative damage regulating mitochondrial iron availability: hypothesis for a protective role in Friedreich ataxia. Hum Mol Genet. 2009;18:1–11.
Richardson DR, Huang ML, Whitnall M, Becker EM, Ponka P, Suryo Rahmanto Y. The ins and outs of mitochondrial iron-loading: the metabolic defect in Friedreich’s ataxia. J Mol Med. 2009;88:323–9.
Acknowledgements
This study was supported by a grant of the Austrian National Bank (ÖNB, Jubiläumsfonds), Medical University Innsbruck, Tiroler Landeskrankenanstalten.
Conflicts of Interest
There is no conflict of interest in the work presented in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nachbauer, W., Hering, S., Seifert, M. et al. Effects of Erythropoietin on Frataxin Levels and Mitochondrial Function in Friedreich Ataxia – a Dose–Response Trial. Cerebellum 10, 763–769 (2011). https://doi.org/10.1007/s12311-011-0287-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-011-0287-9