Abstract
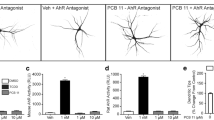
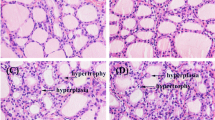
1,2,5,6,9,10-αHexabromocyclododecane (HBCD) is a nonaromatic, brominated cyclic alkane used as an additive flame retardant. It bioaccumulates, persists in the environment, and has been detected in humans and wildlife. Its developmental neurotoxicity is of great concern. We investigated the effect of HBCD on thyroid hormone (TH) receptor (TR)-mediated transcription using transient transfection-based reporter gene assays and found that a low-dose (10−10 M) HBCD suppressed TR-mediated transcription. We further examined the effect of HBCD on interaction of TR with TH response element (TRE) and found a partial dissociation of TR from TRE. HBCD did not dissociate steroid receptor coactivator-1 from TR in the presence of TH; neither did it recruit corepressors (N-CoR and SMRT) to TR in the absence of TH. Furthermore, low-dose HBCD (10−10 M) significantly suppressed TH-induced dendrite arborization of Purkinje cells in primary cerebellar culture derived from newborn rat. These results show that low-dose HBCD can potentially disrupt TR-mediated transactivation and impairs Purkinje cell dendritogenesis, suggesting that HBCD can interfere with TH action in target organs, including the developing brain.






Similar content being viewed by others
References
de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624.
Law RJ, Kohler M, Heeb NV, Gerecke AC, Schmid P, Voorspoels S, et al. Hexabromocyclododecane challenges scientists and regulators. Environ Sci Technol. 2005;39:281A–7A.
Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor TM. Brominated flame retardant concentrations and trends in abiotic media. Chemosphere. 2006;64:181–6.
Law RJ, Herzke D, Harrad S, Morris S, Bersuder P, Allchin CR. Levels and trends of HBCD and BDEs in the European and Asian environments, with some information for other BFRs. Chemosphere. 2008;73:223–42.
van der Ven LT, Verhoef A, van der Kuil T, Slob W, Leonard PE, Visser TJ, et al. A 28-day oral dose toxicity study enhanced to detect the endocrine effects of hexabromocyclododecane in Wister rats. Toxicol Sci. 2006;94:281–92.
Eriksson P, Viberg H, Fischer C, Wallin M, Fredriksson A. A comparison on developmental neurotoxic effects of hexabromocyclododecane,2,2’,4,4’,5,5’-hexabromodiphenylether (PBDE153) and 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB153). Organohalo Comp. 2002;57:389–92.
Germer S, Piersma A, van der Ven L, Kamyschnikow A, Fery Y, Schmitz H, et al. Subacute effects of the brominated flame retardant hexabromocyclododecane and tetrabromobisphenol-A on hepatic cytochrome P450 levels in rats. Toxicology. 2006;218:229–36.
Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat synaptosomes and vesicles. Neurochem Int. 2003;43:533–42.
Yamada-Okabe T, Sakai H, Kashima Y, Yamada-Okabe H. Modulation at a cellular level of the thyroid hormone receptor mediated gene expression by 1,2,5,6,9,10-hexabromocyclododecane (HBCD), 4,4’-diiodobiphenyl (DIB), and nitrofen (NIP). Toxicol Lett. 2005;155:127–33.
Thomsen C, FrØshaug M, Broadwell SL, EggesbØ M. Levels of brominated flame retardants in milk from the Norwegian human milk study: HUMIS. Organohal Comp. 2005;67:509–12.
Shi ZX, Wu YN, Li JG, Zhao YF, Feng JF. Dietary exposure assesment of Chinese adults and nursing infants to tetrabromobisphenol-A and hexabromocyclododecane: occurence measurements in food and human milk. Environ Sci Technol. 2009;43:4314–9.
Potterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurologic development: current perspectives. Endocr Rev. 1993;14:94–106.
Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83.
Koibuchi N, Chin WW. Thyroid hormone action and brain development. Trends Endocrinol Metab. 2000;11:123–8.
Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–142.
Haddow JE, Palomoki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent development of the child. New Engl J Med. 1999;341:549–55.
Bradley DJ, Towle HC, Young WS. Spatial and temporal expression of α- and β-thyroid hormone receptor mRNAs, including the β2-subtype, in the developing mammaliam nervous system. J Neurosci. 1992;12:2288–302.
Koibuchi N. The role of thyroid hormone on cerebellar development. Cerebellum. 2008;7:530–3.
Haukas M, Mauriussen E, Ruus A, Tollesfen KE. Accumulation and disposition of hexabromocyclododecane (HBCD) in juvenile rainbow trout (Oncorhynchus mykiss). Aquatic Toxicology. 2009;95:144–51.
Ito M. Historical review of the significance of the cerebellum and the role of the Purkinje cells in motor learning. Ann NY Acad Sci. 2002;978:273–8.
Hirai H. Progress in transduction of cerebellar Purkinje cells in vivo using viral vectors. Cerebellum. 2008;7:273–8.
Nicholson JL, Altman J. Synaptogenesis in the rat cerebellum: effects of early hypo- and hyperthyroidism. Science. 1972;176:530–2.
Kimura-Kuroda J, Nagata I, Negishi-Kato M, Kuroda Y. Thyroid hormone-dependent development of mouse cerebellar Purkinje cells in vitro. Dev Brain Res. 2002;137:55–65.
Iwasaki T, Chin WW, Ko L. Identification and characterization of RRM-containing coactivator activator (CoAA) as TRBP-interacting protein and its splice variant as a coactivator modulator (CoAM). J Biol Chem. 2001;276:33375–83.
Koibuchi N, Liu Y, Fukuda H, Takeshita A, Yen PM, Chin WW. ROR augments thyroid hormone receptor-mediated transcriptional activation. Endocrinology. 1999;140:1356–64.
Takeshita A, Yen PM, Ikeda M, Cardona GR, Liu Y, Koibuchii N, et al. Thyroid hormone response elements differentially modulate the interactions of thyroid hormone receptor with two receptor binding domain in the steroid receptor coactivator-1. J Biol Chem. 1998;273:21554–62.
Takeshita A, Taguchi M, Koibuchi N, Ozawa Y. Putative role of the orphan nuclear receptor SXR (steroid and xenobiotic receptor) in the mechanism of CYP3A4 inhibition by xenobiotics. J Biol Chem. 2002;277:32453–8.
Iwasaki T, Miyazaki W, Takeshita A, Kuroda Y, Koibuchi N. Polychlorinated biphenyls suppress thyroid hormone-induced transactivation. Biochem Biophys Res Commun. 2002;298:384–8.
Jiang D, Sullivan PG, Sensi SL, Steward O, Wises JH. Zn (2+) induces permeability transition pore opening and release of pro-apoptotic peptodes from neuronal membrane. J Biol Chem. 2001;276:47524–9.
Iwasaki T, Miyazaki W, Rokutanda N, Koibuchi N. Liquid chemiluminescent DNA pull-down assay to measure nuclear receptor-DNA binding in solution. Biotechniques. 2008;45:445–8.
Kimura-Kuroda J, Nagata I, Kuroda Y. Disrupting effects of hydroxlated-polychlorinated biphenyl (PCB) congeners on neuronal development of cerebellar Purkinje cells: a possible causal factor for developmental brain disorders? Chemosphere. 2007;67:412–20.
Miyazaki W, Iwasaki T, Takeshita A, Kuroda Y, Koibuchi N. Polychlorinated biphenyls suppress thyroid hormone receptor-mediated transcription through a novel mechanism. J Biol Chem. 2004;279:18195–202.
Miyazaki W, Iwasaki T, Takeshita A, Tohyama C, Koibuchi N. Identification of the functional domain of thyroid hormone receptor responsible for polychlorinated biphenyl-mediated suppression of its action in vitro. Environ Health Perspect. 2008;116:1231–6.
Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5158–90.
Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149.
Koibuchi N, Jingu H, Iwasaki T, Chin WW. Current perspectives on the role of thyroid hormone in growth and development of the cerebellum. Cerebellum. 2003;2:279–89.
Strait KA, Schwartz HL, Seybold VS, Ling NC, Oppenheimer JH. Immunofluoresecence localization of thyroid hormone receptor protein β1 and α2 in selscted tissues: cerebellar Purkinje cells as a model for β1 receptor-mediated developmental effects of thyroid hormone in brain. Proc Natl Acad Sci USA. 1991;88:3887–91.
Acknowledgement
We thank Drs. W. Miyazaki, J. Kimura-Kuroda, T. Aoki, K. Takata, K. Hanamura, and T. Shirao for helpful assistance, and Dr. A. Takeshita for kindly providing plasmids. This project was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (17510039, T.I, 17390060, N.K), and a grant from Ministry of the Environment of Japan (to N.K, T.I, and N.S).
Competing interest
All authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ibhazehiebo, K., Iwasaki, T., Shimokawa, N. et al. 1,2,5,6,9,10-αHexabromocyclododecane (HBCD) Impairs Thyroid Hormone-Induced Dendrite Arborization of Purkinje Cells and Suppresses Thyroid Hormone Receptor-Mediated Transcription. Cerebellum 10, 22–31 (2011). https://doi.org/10.1007/s12311-010-0218-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-010-0218-1