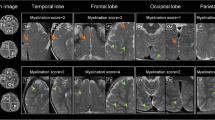
Abstract
Age-dependent changes in the normal cerebral white matter have been reported; however, there is no study on normal cerebellar white matter maturation in developing brain using diffusion tensor imaging (DTI). We performed DTI in 21 children who had normal neurological assessment along with no evidence of any abnormality on imaging. The aim of this study was to compare the age-related changes in fractional anisotropy (FA) and mean diffusivity (MD) quantified from cerebral white matter (splenium and genu of the corpus callosum and posterior limb of the internal capsule) and cerebellar white matter (middle cerebellar peduncles, superior cerebellar peduncles, and inferior cerebellar peduncles) regions in healthy children ranging in age from birth to 132 months. Log-linear regression model showed best fit to describe the age-related changes in FA and MD both for cerebral and cerebellar white matter. In cerebral white matter, an initial sharp increase in FA was observed up to the age of 24 months followed by a gradual increase up to 132 months. In cerebellar white matter, sharp increase in FA was observed up to 36 months, which then followed a gradual increase. However, MD showed a sharp decrease in cerebral white matter up to 24 months followed by a more gradual decrease thereafter, while in cerebellar white matter after an initial decrease (6 months), it followed a stable pattern. This study provides normative database of brain white matter development from neonates to childhood. This quantitative information may be useful for assessing brain maturation in patients with developmental delay of the cerebral and cerebellar white matter.



Similar content being viewed by others
References
Yakovlev PI, Lecours AR (1967) The myelogenetic cycles of regional maturation of the brain. In: Minkowski A (ed) Regional development of the brain in early life. Oxford, England, Blackwell Scientific, pp 3–70
Ricardson EP Jr (1982) Myelination in the human central nervous system. In: Haymaker W, Adams RD (eds) Histology and histopathology of the nervous system. Springfield, III, Thomas, pp 146–73
Barkovich AJ (2000) Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol 21:1099–1109
Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A (2001) Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull 54:255–266
Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK (2005) Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp 26:139–147
Paus T, Zijdenbos A, Worsley K, Collins L, Blumenthal J, Giedd JN et al (1999) Structural maturation of neural pathways in children and adolescents: in vivo study. Science 283:1908–1911
Brody BA, Kinney HC, Kloman AS, Gilles FH (1987) Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol 46:283–301
Kinney HC, Brody BA, Kloman AS, Gilles FH (1988) Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol 47:217–234
Gilles FH, Shankle W, Dooling EC (1983) Myelinated tracts: growth patterns. In: Gilles FH, Leviton A, Dooling EC (eds) The developing human brain. PSG, Boston, John Wright, pp 117–183
Barkovich AJ, Kjos BO, Jackson DE Jr, Norman D (1988) Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology 166:173–180
van der Knaap MS, Valk J (1990) MR imaging of the various stages of normal myelination during the first year of life. Neuroradiology 31:459–470
Barkovich AJ (ed) (2005) Pediatric neuroimaging, 4th edn. Lippincott, Philadelphia
Mukherjee P, McKinstry RC (2006) Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am 16:19–43
Wimberger DM, Roberts TP, Barkovich AJ, Prayer LM, Moseley ME, Kucharczyk J (1995) Identification of “premyelination” by diffusion-weighted MRI. J Comput Assist Tomogr 19:28–33
Prayer D, Barkovich AJ, Kirschner DA, Prayer LM, Roberts TP, Kucharczyk J et al (2001) Visualization of nonstructural changes in early white matter development on diffusion-weighted MR images: evidence supporting premyelination anisotropy. AJNR Am J Neuroradiol 22:1572–1576
Nomura Y, Sakuma H, Takeda K, Tagami T, Okuda Y, Nakagawa T (1994) Diffusional anisotropy of the human brain assessed with diffusion-weighted MR: relation with normal brain development and aging. AJNR Am J Neuroradiol 15:231–238
Morriss MC, Zimmerman RA, Bilaniuk LT, Hunter JV, Haselgrove JC (1999) Changes in brain water diffusion during childhood. Neuroradiology 41:929–934
Kizildağ B, Düşünceli E, Fitoz S, Erden I (2005) The role of classic spin echo and FLAIR sequences for the evaluation of myelination in MR imaging. Diagn Interv Radiol 11:130–136
Murakami JW, Weinberger E, Shaw DW (1999) Normal myelination of the pediatric brain imaged with fluid-attenuated inversion-recovery (FLAIR) MR imaging. AJNR Am J Neuroradiol 20:1406–1411
Engelbrecht V, Rassek M, Preiss S, Wald C, Modder U (1998) Age-dependent changes in magnetization transfer contrast of white matter in the pediatric brain. AJNR Am J Neuroradiol 19:1923–1929
van Buchem MA, Steens SC, Vrooman HA, Zwinderman AH, McGowan JC, Rassek M et al (2001) Global estimation of myelination in the developing brain on the basis of magnetization transfer imaging: a preliminary study. AJNR Am J Neuroradiol 22:762–766
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15:435–455
Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CE et al (1998) Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology 209:57–66
Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA et al (1998) Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res 44:584–590
McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL et al (2002) Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex 12:1237–1243
Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR et al (2001) Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology 221:349–358
McGraw P, Liang L, Provenzale JM (2002) Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. AJR Am J Roentgenol 179:1515–1522
Schneider JF, Il’yasov KA, Hennig J, Martin E (2004) Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology 46:258–266
Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC et al (2006) Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage 29:493–504
Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D (2006) Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage 30:1121–1132
Le Bihan D (ed) (1995) Diffusion and perfusion MRI—applications to functional MRI. Raven, New York
Hasan KM, Parker DL, Alexander AL (2001) Comparison of gradient encoding schemes for diffusion-tensor MRI. J Magn Reson Imaging 13:769–780
Woods RP, Mazziotta JC, Cherry SR (1993) MRI–PET registration with automated algorithm. J Comput Assist Tomogr 17:536–546
Purwar A, Gupta RK, Sarma MK, Bayu G, Singh A, Rathore DK et al (2006) De-scalping of the brain in echo planar DT-MRI. Proceedings of the International Society of Magnetic Resonance in Medicine, p 325
Hasan KM, Basser PJ, Parker DL, Alexander AL (2001) Analytical computation of the eigenvalues and eigenvectors in DT-MRI. J Magn Reson 152:41–47
Purwar A, Rathore DK, Rathore RKS, Gupta RK (2006) A DTI analysis tool. Proceedings of the European Society of Magnetic Resonance in Medicine, Abstract 644
Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S (2004) Fiber tract-based atlas of human white matter anatomy. Radiology 230:77–87
Dietrich RB, Bradley WG, Zaragoza EJ IV, Otto RJ, Taira RK, Wilson GH et al (1988) MR evaluation of early myelination patterns in normal and developmentally delayed infants. AJR Am J Roentgenol 150:889–896
Benes FM, Turtle M, Khan Y, Farol P (1994) Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry 51:477–484
Dobbing J, Sands J (1973) Quantitative growth and development of human brain. Arch Dis Child 48:757–767
Engelbrecht V, Scherer A, Rassek M, Witsack HJ, Mödder U (2002) Diffusion-weighted MR imaging in the brain in children: findings in the normal brain and in the brain with white matter diseases. Radiology 222:410–418
Stricker T, Martin E, Boesch C (1990) Development of the human cerebellum observed with high-field-strength MR imaging. Radiology 177:431–435
Schmahmann JD (2004) Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 16:367–378
Acknowledgment
This study was supported by grant no. BT/PR5009/Med/14/581/2004 from the Department of Biotechnology, New Delhi, India. Sona Saksena acknowledges the financial assistance from the Indian Council of Medical Research, New Delhi, India. Richa Trivedi acknowledges the financial assistance from the Council of Scientific and Industrial Research, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saksena, S., Husain, N., Malik, G.K. et al. Comparative Evaluation of the Cerebral and Cerebellar White Matter Development in Pediatric Age Group using Quantitative Diffusion Tensor Imaging. Cerebellum 7, 392–400 (2008). https://doi.org/10.1007/s12311-008-0041-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-008-0041-0