Abstract
On average, 30% of patients with myelodysplastic syndrome (MDS) develop overt acute myeloid leukemia (AML) during the course of the disease. There is a continuous search for the best model of individual risk assessment for MDS patients. In this review, we summarize current findings on factors that have been associated with increased risk of AML transformation. These include laboratory values such as high lactate dehydrogenase levels, complex karyotypes, numbers and aberrant immunophenotype of bone marrow blasts, bone marrow-related features such as numbers and distribution of CD34+ cells, and recently established molecular markers. A wide range of described molecular aberrations in MDS, including various gene mutations, chromosomal instability, short telomeres, high levels of gene methylation, and histone modification, partly explains clinical heterogeneity of this disease. Continuous research will bring more insight in the pathogenesis of various MDS categories, making individual risk assessment and tailored therapy for each patient possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Myelodysplastic syndromes (MDS) are a heterogeneous group of clonal stem cell disorders characterized by an extremely variable clinical course ranging from indolent disease with only minimal impact on survival of elderly patients to a very aggressive course with quick progression to acute leukemia [1]. On average, 30% of MDS patients develop overt acute myeloid leukemia (AML) during the course of the disease. The World Health Organization (WHO) classification identifies several MDS subtypes with a variable risk of AML transformation [1–3] (Table 1). There is a continuous search for the best model of individual risk-assessment for MDS patients. Several risk-related factors have been described, including clinical features, laboratory values, cytogenetics, bone marrow-related features, and recently established molecular markers [2, 4–8].
Mechanisms of disease progression and transformation from a chronic MDS phase to a more aggressive AML phase are still poorly understood. A mechanism based on an accumulation of cytogenetic and molecular aberrations during the course of disease, following a multi-step model of leukemogenesis, has been proposed (Fig. 1) [8]. However, most recently detected molecular changes occur only in a fraction of MDS patients and no universal molecular mechanism has as yet been found. The balance between apoptosis and proliferation within hematopoiesis, host-response related features, and stroma defects seem also to play a role.
Two-hit model of transformation from MDS to AML. Class I targeted genes are involved in signal transduction, while mutations in Class II genes affect transcription factors. Both Class I and Class II mutations can also lead to de novo AML [8]
In the following review, we summarize current findings from clinical, laboratory, morphological, phenotypical, immunopathological, cytogenetic, and molecular research as related to the transformation from MDS to AML.
Prognostic scoring systems and assessment of risk for MDS–AML transformation
The International Prognostic Scoring System (IPSS), published in 1997, was based on the percentage of blasts in the bone marrow (BM) smear, number of cytopenias (defined as hemoglobin (Hb) levels less than 10 g/l, an absolute neutrophil count (ANC) of less than 1.5 × 109/L, and a platelet count of less than 100 × 109/L), and cytogenetic findings [5]. The IPSS could be applied to predict risk of AML transformation; 19% of low-risk patients, 30–33% of intermediate-risk, and 45% of high-risk patients died of AML in the primary cohort of Greenberg et al. [5] (Table 2). The clinical significance of IPSS has been verified in several studies [9–11] that also found IPSS to be of high predictive value for AML transformation [12]. Germing et al. [2] reported in 2005 that the addition of the lactate dehydrogenase (LDH) level to IPSS improved its prognostic value. MDS patients with elevated LDH had significantly higher risk of AML transformation. Malcovati et al. [6] proposed a prognostic scoring system based on the WHO2001 classification of MDS, cytogenetic findings, and transfusion requirements (WPSS). The WPPS defined five risk categories where very low-risk patients had only a 3% cumulative probability of AML transformation within 2 and 5 years, and for very high-risk patients, the respective probability was 80% and 84% (Table 2). The most recent MD Anderson prognostic scoring system is also based on the percentages of BM blasts and cytogenetics, but it does not take the WHO categories into consideration [7]. This system introduces other clinical data such as age, performance status, and transfusion requirements and applies a more detailed assessment of peripheral blood values, i.e., Hb, white blood cell count, and platelet levels. The MD Anderson system identifies low-risk patients in the IPSS high-risk group and patients with poorer survival within the IPPS low-risk group. However, no data concerning the assessment of the risk of transformation has been presented. Age ≥65 is considered as an adverse prognostic factor by the MD Anderson system. However, Nösslinger et al. [13] reported that within the IPSS high-risk category, women <65 years of age had the worst prognosis.
Morphological and immunophenotypic features related to disease progression in MDS
All above-mentioned prognostic scores include the number of BM blast evaluated in BM smears as a significant prognostic factor for AML transformation risk (Table 2). An arbitrary limit of 5% blasts has been used to define patients with refractory anemia with excess of blasts (RAEB) [1, 14]. A recent Chinese study indicated that refractory cytopenia with multilineage dysplasia (RCMD) patients with >3.5% blasts may have worse outcome [15]. Knipp et al. [16] reported the prognostic significance of increased peripheral blasts in patients with <5% BM blasts. Patients (16.2%) with at least 1% peripheral blasts suffered AML transformation by comparison to 6.8% of patients with less than 1% blasts in blood. Therefore, patients with ≥1% peripheral blasts have been included in the RAEB category in the 2008 WHO classification [1]. Several studies indicated that patients with multilineage dysplasia had a higher risk of AML transformation compared to those with unilineage dysplasia, which in part may be related to the higher incidence of multilineage dysplasia in patients with chromosomal changes [3, 17–19].
Aberrant phenotypes in BM blast population such as expression of lymphoid-associated markers CD7 (Fig. 2), CD4, and CD56 have been more often seen in MDS in transformation [20]. The aberrant expression of CD7 [20], overexpression of CD34 and CD36 [21], expression of Tdt [22], and the accumulation of phenotypic aberrancies, so called high flow score [23], have also been associated with the progression of disease in MDS.
Example of an emerging pathological blast population demonstrated by flow cytometry in a bone marrow from MDS patient. CD34+ cells are gated on CD34/Side scatter plot (upper right). Lower left plot shows that the population of CD34+ blasts partly displays an aberrant expression of CD7 (red dots). These cells can be found in the blast region (CD45 dim, left upper plot) and are only weakly CD33 positive (right lower plot). CD34+ CD7+ cells are not positive for CD56, but there is an increased CD56 expression in a subpopulation of granulopoietic precursors (right lower plot)
The extent of blast infiltration has also been studied in BM biopsies by morphology and by CD34 immunohistochemistry (Fig. 3) that helps to evaluate topographic distribution of blasts [24–26]. Presence of abnormally localized immature precursors (ALIPs) and clusters of CD34 positive cells significantly increased risk of AML transformation, independent of IPSS. ALIPs and CD34 clusters were more often seen in MDS patients with RAEB. Causes of deaths in MDS patients who displayed CD34+ clusters were mostly related to leukemic transformation. The increased expression of CD34 in megakaryocytes was reported as a negative prognostic factor [27]. Clusters of CD117+ cells have also been described as ALIPs, but their significance in relation to AML transformation has not yet been determined [28].
Patients with high BM cellularity have a higher risk of transformation when compared to those with hypoplastic MDS [29]. Significant fibrosis detected by reticulin staining of BM biopsies from MDS patients also indicates an increased risk of AML transformation, independent of other variables [26, 30]. Literature data concerning a possible role of BM stroma-related factors in MDS–AML transformation is scarce. A variety of stromal defects may be present in various MDS subgroups, and some of these may facilitate transformation to AML [31]. Recent studies have shown that stromal factors can increase the susceptibility of stem cells to apoptosis in some MDS patients [32]. Fibroblasts derived from some MDS marrows, produced significantly higher levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). Macrophages from these MDS patients produced significantly higher levels of TNF-α than their normal counterparts [33]. Other MDS patients may have normal BM stroma. Since it has been demonstrated that following successful transplantation, fibroblasts (stroma cells) remain of host origin [34], MDS patients with normal BM stroma may have better chance to respond well to stem cell transplantation [35]. In MDS patients with del(5q), bone marrow stroma shows a decreased capacity to support hematopoiesis, which could be reversed by lenalidomide treatment [36].
Also, aberrant expression of vascular endothelial growth factor (VEGF) has been demonstrated in ALIPs of BM biopsies taken from MDS patients. It has been suggested that VEGF may provide signals reinforcing leukemia cell survival and this way contribute to transformation [37].
Cytogenetics
There is a consensus in the literature that complex karyotypes characterize a group of MDS patients with poor prognosis and a high-risk of transformation to AML [reviewed in [38]]. Most prognostic scoring systems consider MDS patients with three or more cytogenetic abnormalities within the same clone as having a complex karyotype while patients with five or more abnormalities are considered as a poor prognosis category in Medical Research Council AML trials. Within the group of MDS patients with complex karyotype, monosomal karyotype defined as the presence of two or more distinct autosomal monosomies or a single monosomy associated with a structural abnormality was related to poorer prognosis and shorter leukemia-free survival [39].
It has been shown that complex karyotypes arise by stepwise accumulation of chromosomal changes and that chromosomal instability of CD34+ population precedes transformation to AML [40]. In the study of Bernasconi et al. [41], 77% of sequentially tested MDS patients who show cytogenetic evolution progress to AML in comparison to 30% of those patients without cytogenetic evolution. In a recent large study of patients with 5q- abnormality, there was a clear correlation between karyotype complexity and the risk of transformation. Patients with isolated 5q- had a 21.1% cumulative probability of AML evolution after 5 years, and patients with 5q- + 1 abnormality had 57.6% probability. Patients with 5q- and two or more aberrancies had a 100% risk of transformation [42]. Also, it has been shown that patients with del(5q) MDS who failed to achieve sustained cytogenetic remission after treatment with lenalidomide have an increased risk of clonal evolution and AML transformation [43]. Most patients who developed AML acquired complex karyotypes.
There was no similar correlation for patients with abnormalities of chromosome 7, which at least in adults carry a high risk of AML transformation, independent of the presence of other abnormalities [38]. A high risk of AML development in these patients may be related to up-regulation of genes which have been implicated in leukemic transformation, such as HOX9A, BRCA2, PRAME, BMI-1, and PLAB. Other up-regulated genes included genes promoting cell proliferation, such as cell cycle regulator SPHAR, the DNA replication check-point gene Rad17, and signal transduction gene TPO [44].
Molecular mechanisms of transformation
Two classes of molecular changes play a role in the development of MDS and transformation to AML (Fig. 1). Class I targeted genes are mostly involved in signal transduction and class II molecular changes affect transcription factors (Table 3, reviewed in [8]). Class II mutations [such as mutations of Tet2, RUNX1, or RSP14 haploinsufficiency in del(5q)] affect cellular differentiation but are probably insufficient to induce AML transformation but play a role in MDS initiation [45, 46]. Class I mutations (such as Flt3, RAS, KIT, IDH1, and NPM1) affect cell proliferation and survival and have been mostly found in MDS patients at progression to AML [8]. When several mutations were investigated in a cohort of MDS patients and AML patients without a complex karyotype or balanced translocation, mutations in the ASXL1 and CBL genes were frequent in RAEB MDS patients. Mutations in the TET2 gene were found both in MDS and in AML and could be associated with either ASXL1 or NPM1 mutations but not with a RUNX1 mutation. The latter could be combined with mutations in the ASXL1 but not the NPM1 gene. Mutations in FLT3, IDH1, IDH2, NPM1, and WT1 were found mainly in AML patients [47]. However, mutations of each of the above-mentioned genes occur only in a relatively small fraction of MDS patients, which points out the heterogeneity of the disease.
Overexpression of the Wilms Tumor gene (WT1) has been reported in MDS [48]. Longitudinal studies have shown that an increase in WT1 mRNA levels in blood was a strong predictor of a short period of time to AML transformation, independent of IPSS [49]. Interestingly, patients with high levels of anti-WT1 antibody had a significantly longer survival.
TP53 mutations have been mainly reported in high-risk and therapy-related MDS and more often in patients with complex karyotypes [50, 51]. An increased expression of p53 protein as detected by the immunohistochemistry in myeloid cells in the BM of MDS patients has been shown to precede AML transformation [52] and to correlate with TP53 mutations [53]. In a recent study using sensitive deep-sequencing technology, small TP53 mutated populations (median clone size 11%) could be demonstrated already at the time of diagnosis in 18% of patients with del(5q) MDS category [54]. These mutations were present years before disease progression and were associated with an increased risk of transformation to AML with 5-year cumulative incidence of leukemic evolution: 77% and 24% in mutated and un-mutated groups of patients, respectively. In patients with TP53 mutations, small numbers of p53 strongly positive cells in the BM could be demonstrated at diagnosis and increased at the time of progression (Fig. 4), indicating the expansion of TP53 mutated population [54]. These results indicate that patients with early stages of MDS can harbor sub-clones that can be resistant to therapy and give rise to AML. The TP53 mutated patients did not achieve complete cytogenetic response to lenalidomide, which indicates therapy resistance. However, AML transformation occurred also in patients without TP53 mutation, suggesting other mechanisms of progression.
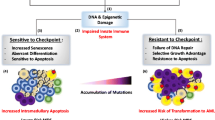
Global gene expression studies combined with detailed annotated pathway analyses and gene oncology analyses have identified multiple deregulated pathways in CD34+ hematopoietic stem cells in MDS patients [55]. In general, the most down-regulated pathway in MDS was the Wnt canonical pathway, which may lead to defective self-renewal of hematopoietic stem cells. The most up-regulated pathways were the “interferon signaling” pathway, which may be responsible for cytopenia and the “thrombopoietin signaling” pathway, which may be one of the causes of megakaryocyte abnormalities and thrombocytopenia. Major differences were found between low-risk MDS cases where most deregulated pathways were related to immune response and apoptosis and high-risk MDS where the most deregulated pathways were related to cell cycle check and DNA repair [55]. The results of this study support a model for MDS in which immune deregulation and apoptosis dominates in early MDS leading to ineffective hamatopoiesis while disruption of DNA damage check-points and increased genomic instability characterize advanced MDS leading to AML transformation (Fig. 5).
Another sign of genetic instability in MDS is reported shortened telomere length, especially in patients with complex cytogenetic abnormalities and advanced disease [56, 57]. If telomeres reach a critically short length, they may form dicentric chromosomes and undergo a break-fusion-bridge cycle that may lead to further genomic changes. A recent study applying the novel technique of combining chromosome banding and T/C-FISH showed that in MDS patients, telomere lengths were shorter in both normal and aberrant metaphases [58]. These results suggest that telomere shortening may be characteristic of all hematopoietic stem cells in MDS or that telomere shortening may be a predisposing factor for development of MDS. In MDS patients who accumulate further molecular changes providing a proliferative advantage, clonal cells may stabilize the aberrations by up-regulation of telomerase activity or other telomere-elongating mechanisms [58].
Epigenetic mechanisms in MDS progression
Epigenetic changes have been recognized in the past decade as major drivers prompting malignant phenotypes [reviewed in [59]]. There are three general molecular mechanisms carrying epigenetic information: DNA methylation, histone modifications, and RNA interference. Aberrant DNA methylation is catalyzed by DNA methyltransferases and occurs within “CpG islands” found in the promoter regions of >50% of human genes. “CpG islands” are CpG dinucleotide-rich regions with lengths of 4 kb or more. Hypermethylation of CpG islands of tumor suppressor genes is probably a progressive process that can confer a selective advantage for the survival of the transformed cell [60]. In MDS, aberrant methylation was seen in every studied BM sample, and the number of methylated CpG sites was significantly greater in high-risk MDS and MDS/AML in comparison to low-risk MDS [61]. Five genes were hypermethylated in more that 70% of patients (ALOX12, GSTM1, HIC1, FZD9, and HS3ST2). Of those, the FZD9 gene methylation was an independent predictor of decreased survival. Clinical outcome was poorest in patients with chromosome 7 deletion and aberrant methylation of the remaining allele [61]. Another study reported that methylation of the CTNNA1 gene promoter was found in 31% patients with AML with del(5q) but not in low-risk del(5q) MDS[62]. The CTNNA1 gene has been suggested as one of the candidate 5q tumor suppressor genes. Thus, in cases of chromosomal deletion, the aberrant DNA methylation of the remaining chromosome may silence the remaining allele of a recessive tumor suppressor gene and increase the risk of transformation.
Another target for methylation analysis in MDS is the inhibitor of DNA binding/inhibitor of differentiation gene (ID). The ID proteins form hetero-dimers with transcription factors and act as the dominant negative inhibitors of gene transcription [63]. Methylation of ID4, which is a putative tumor suppressor gene, has been found in 35.1% of MDS patients and patients with methylated ID4 progressed to AML more rapidly that those without methylation[64].
Hypermethylation of the CDNK2B (p15 INKAB) gene found at diagnosis in patients with low-risk MDS has also been strongly associated with AML transformation [65]. In a recent study, a prognostic significance of a combination of methylation profiles of ten selected genes was evaluated and the high methylation score was shown to be an independent predictor of shorter progression-free survival [66].
Histones are small proteins that form a core around which DNA is wrapped, forming nucleosomes. The best understood histone modifications are acetylation and methylation of specific residues. Histone modifications form a code that integrates gene activation/inactivation/silencing signals, so that transcriptional activity of a given promoter can be predicted by looking at the specific histone modifications [59]. Specific studies of histone modifications in MDS are rare, but mutations in the EZH2 gene that codes for histone methyltrasferase at chr.7q36.1 have been described [67]. There are a variety of histone deacetylase (HDAC) inhibitors (HDIs) in clinical trials as well as some drugs that can inhibit the activity of histone methyltransferases [68, 69].
Apoptosis and proliferation
Several studies have shown increased rates of programmed cell death (PCD, apoptosis) in the BM of MDS patients [reviewed in [70]]. Increased apoptosis, which exceeds proliferation rate, is mainly considered as a characteristic of the early-stage disease, while disease progression is associated with a reduction in apoptosis [71]. Several cytokines have been shown to be overexpressed in MDS, including TNF-α, transforming growth factor (TGF)-β, interferon (IFN)-γ, IL-6, and IL-1β [70]. TNF-α may be produced by macrophages [72] and the levels of TNF-α in BM plasma have been shown to correlate with pro-apoptotic Fas expression and apoptosis [73, 74]. TNF-α levels were lower in MDS patients with RAEB than in RA/RA-RS, which suggests a decreased role of TNF-α at progression. Other overexpressed molecules acting as negative regulators of hematopoiesis in MDS include FAS-ligand and TRAIL with their respective agonistic receptors [70]. At progression, there is a shift of balance in the signals and antiapoptotic/pro-proliferative signals prevail, including increased expression of Bcl-2 (Fig. 5) [71, 75].
Moreover, CD34+ BM cells from high-risk MDS patients or at progression showed higher levels of NF-κB compared to the early-stage of the disease. High NF-κB activity could lead to up-regulation of FLIP (FLICE-inhibitory protein) and other NF-κB dependent antiapoptotic regulators (such as Bcl-xL, Bcl-2, XIAP) followed by increased resistance of CD34+ cells to apoptosis in these patients [76].
Autoimmunity and host response
Several observations suggest that immune dysregulation plays a role in the pathogenesis of low-risk MDS and contributes to ineffective hematopoiesis in these patients. A proposed model of immune pathogenesis implied that CD8+ cells are stimulated by unique or overexpressed antigens, which leads to T cell receptor repertoire contraction through expansion of memory cells and repression of hematopoiesis trough cross-reactive antigens expressed on normal BM progenitors [77]. Both CD4 and CD8 subsets seem to be dysregulated in MDS. In younger MDS patients, reduced levels of naïve CD4+ T cells were associated with response to immunosuppressive therapy [78]. It has been suggested that loss of CD4+ cells in MDS may affect mainly regulatory T cells (T-regs), which are important for peripheral tolerance and prevention of an autoimmune process, while the numbers of CD3+ CD4+ IL-17 producing T cells were increased [79]. The levels of T regs in blood of high-risk MDS patients were higher than in the low-risk group and the levels further increased in patients who progressed to leukemia [80]. Also T-regs in patients with advanced MDS retained their functional capacity in contrast to T-regs from low-risk MDS patients that had a reduced ability to suppress immune responses. Moreover, patients with high-risk MDS have reduced natural killer (NK) cell function and reduced expression of activating NK receptors [81]. Thus, in high-risk MDS patients, impaired immune surveillance may contribute to progression and leukemic evolution.
Conclusions
Although great progress have been made in understanding molecular pathogenesis of MDS, the exact defects that make some patients quickly transform to aggressive and usually therapy-resistant AML phase are often not clear and are probably complex. Continuous research will bring more insight in the pathogenesis of various MDS categories, making individual risk assessment and tailored therapy for each patient possible.
References
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (2008) WHO classification of tumours of haematopoietic and lymphatic tissues. IARC, Lyon
Germing U, Strupp C, Kuendgen A, Isa S, Knipp S, Hildebrandt B, Giagounidis A, Aul C, Gattermann N, Haas R (2006) Prospective validation of the WHO proposals for the classification of myelodysplastic syndromes. Haematologica 91:1596–1604
Breccia M, Latagliata R, Cannella L, Carmosino I, Santopietro M, Loglisci G, Federico V, Alimena G (2010) Refractory cytopenia with unilineage dysplasia: analysis of prognostic factors and survival in 126 patients. Leuk Lymphoma 51:783–788
Mufti GJ, Stevens JR, Oscier DG, Hamblin TJ, Machin D (1985) Myelodysplastic syndromes: a scoring system with prognostic significance. Br J Haematol 59:425–433
Greenberg PL (1998) Risk factors and their relationship to prognosis in myelodysplastic syndromes. Leuk Res 22(Suppl 1):S3–S6
Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, Giagounidis A, Hildebrandt B, Bernasconi P, Knipp S, Strupp C, Lazzarino M, Aul C, Cazzola M (2007) Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 25:3503–3510
Kantarjian H, O'Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, List A, Fenaux P, Sanz G, Issa JP, Freireich EJ, Garcia-Manero G (2008) Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer 113:1351–1361
Nolte F, Hofmann WK (2010) Molecular mechanisms involved in the progression of myelodysplastic syndrome. Future Oncol 6:445–455
Hellstrom-Lindberg E, Ahlgren T, Beguin Y, Carlsson M, Carneskog J, Dahl IM, Dybedal I, Grimfors G, Kanter-Lewensohn L, Linder O, Luthman M, Lofvenberg E, Nilsson-Ehle H, Samuelsson J, Tangen JM, Winqvist I, Oberg G, Osterborg A, Ost A (1998) Treatment of anemia in myelodysplastic syndromes with granulocyte colony-stimulating factor plus erythropoietin: results from a randomized phase II study and long-term follow-up of 71 patients. Blood 92:68–75
Maes B, Meeus P, Michaux L, Bijnens L, Boogaerts M, Hagemeijer A, de Wolf-Peeters C, Verhoef G (1999) Application of the International Prognostic Scoring System for myelodysplastic syndromes. Ann Oncol 10:825–829
Belli C, Acevedo S, Bengio R, Arrossagaray G, Watman N, Rossi N, Garcia J, Flores G, Goldztein S, Larripa I (2002) Detection of risk groups in myelodysplastic syndromes. A multicenter study. Haematologica 87:9–16
Sperr WR, Wimazal F, Kundi M, Fonatsch C, Thalhammer-Scherrer R, Schernthaner GH, Schwarzinger I, Haas OA, Geissler K, Lechner K, Valent P (2001) Survival analysis and AML development in patients with de novo myelodysplastic syndromes: comparison of six different prognostic scoring systems. Ann Hematol 80:272–277
Nosslinger T, Tuchler H, Germing U, Sperr WR, Krieger O, Haase D, Lubbert M, Stauder R, Giagounidis A, Valent P, Pfeilstocker M (2010) Prognostic impact of age and gender in 897 untreated patients with primary myelodysplastic syndromes. Ann Oncol 21:120–125
Tennant GB, Al-Sabah AI, Burnett AK (2002) Prognosis of myelodysplasic patients: non-parametric multiple regression analysis of populations stratified by mean corpuscular volume and marrow myeloblast number. Br J Haematol 119:87–96
Wang H, Wang XQ, Xu XP, Lin GW (2009) Bone marrow blasts level predicts prognosis in patients with refractory cytopenia with multilineage dysplasia. Eur J Haematol 83:550–558
Knipp S, Strupp C, Gattermann N, Hildebrandt B, Schapira M, Giagounidis A, Aul C, Haas R, Germing U (2008) Presence of peripheral blasts in refractory anemia and refractory cytopenia with multilineage dysplasia predicts an unfavourable outcome. Leuk Res 32:33–37
Howe RB, Porwit-MacDonald A, Wanat R, Tehranchi R, Hellstrom-Lindberg E (2004) The WHO classification of MDS does make a difference. Blood 103:3265–3270
Verburgh E, Achten R, Louw VJ, Brusselmans C, Delforge M, Boogaerts M, Hagemeijer A, Vandenberghe P, Verhoef G (2007) A new disease categorization of low-grade myelodysplastic syndromes based on the expression of cytopenia and dysplasia in one versus more than one lineage improves on the WHO classification. Leukemia 21:668–677
Breccia M, Carmosino I, Biondo F, Mancini M, Russo E, Latagliata R, Alimena G (2006) Usefulness and prognostic impact on survival of WHO reclassification in FAB low risk myelodyplastic syndromes. Leuk Res 30:178–182
Ogata K, Nakamura K, Yokose N, Tamura H, Tachibana M, Taniguchi O, Iwakiri R, Hayashi T, Sakamaki H, Murai Y, Tohyama K, Tomoyasu S, Nonaka Y, Mori M, Dan K, Yoshida Y (2002) Clinical significance of phenotypic features of blasts in patients with myelodysplastic syndrome. Blood 100:3887–3896
Maynadie M, Picard F, Husson B, Chatelain B, Cornet Y, Le RG, Campos L, Dromelet A, Lepelley P, Jouault H, Imbert M, Rosenwadj M, Verge V, Bissieres P, Raphael M, Bene MC, Feuillard J (2002) Immunophenotypic clustering of myelodysplastic syndromes. Blood 100:2349–2356
Font P, Subira D, Mtnez-Chamorro C, Castanon S, Arranz E, Ramiro S, Gil-Fernandez JJ, Lopez-Pascual J, Alonso A, Perez-Saenz MA, Alaez C, Renedo M, Blas C, Escudero A, Fdez-Ranada JM (2006) Evaluation of CD7 and terminal deoxynucleotidyl transferase (TdT) expression in CD34+ myeloblasts from patients with myelodysplastic syndrome. Leuk Res 30:957–963
van de Loosdrecht AA, Westers TM, Westra AH, Drager AM, van der Velden VH, Ossenkoppele GJ (2008) Identification of distinct prognostic subgroups in low- and intermediate-1-risk myelodysplastic syndromes by flow cytometry. Blood 111:1067–1077
Soligo DA, Oriani A, Annaloro C, Cortelezzi A, Calori R, Pozzoli E, Nosella D, Orazi A, Deliliers GL (1994) CD34 immunohistochemistry of bone marrow biopsies: prognostic significance in primary myelodysplastic syndromes. Am J Hematol 46:9–17
Verburgh E, Achten R, Maes B, Hagemeijer A, Boogaerts M, de Wolf-Peeters C, Verhoef G (2003) Additional prognostic value of bone marrow histology in patients subclassified according to the International Prognostic Scoring System for myelodysplastic syndromes. J Clin Oncol 21:273–282
Della Porta MG, Malcovati L, Boveri E, Travaglino E, Pietra D, Pascutto C, Passamonti F, Invernizzi R, Castello A, Magrini U, Lazzarino M, Cazzola M (2009) Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J Clin Oncol 27:754–762
Tang G, Wang SA, Menon M, Dresser K, Woda BA, Hao S (2011) High-level CD34 expression on megakaryocytes independently predicts an adverse outcome in patients with myelodysplastic syndromes. Leuk Res 35(6):766–770
Naresh KN, Lampert IA (2006) CD117 expression as an aid to identify immature myeloid cells and foci of ALIP in bone marrow trephines. Am J Hematol 81:79
Huang TC, Ko BS, Tang JL, Hsu C, Chen CY, Tsay W, Huang SY, Yao M, Chen YC, Shen MC, Wang CH, Tien HF (2008) Comparison of hypoplastic myelodysplastic syndrome (MDS) with normo-/hypercellular MDS by International Prognostic Scoring System, cytogenetic and genetic studies. Leukemia 22:544–550
Buesche G, Teoman H, Wilczak W, Ganser A, Hecker H, Wilkens L, Gohring G, Schlegelberger B, Bock O, Georgii A, Kreipe H (2008) Marrow fibrosis predicts early fatal marrow failure in patients with myelodysplastic syndromes. Leukemia 22:313–322
Tauro S, Hepburn MD, Bowen DT, Pippard MJ (2001) Assessment of stromal function, and its potential contribution to deregulation of hematopoiesis in the myelodysplastic syndromes. Haematologica 86:1038–1045
Mhyre AJ, Marcondes AM, Spaulding EY, Deeg HJ (2009) Stroma-dependent apoptosis in clonal hematopoietic precursors correlates with expression of PYCARD. Blood 113:649–658
Flores-Figueroa E, Montesinos JJ, Flores-Guzman P, Gutierrez-Espindola G, Arana-Trejo RM, Castillo-Medina S, Perez-Cabrera A, Hernandez-Estevez E, Arriaga L, Mayani H (2008) Functional analysis of myelodysplastic syndromes-derived mesenchymal stem cells. Leuk Res 32:1407–1416
Ramakrishnan A, Awaya N, Bryant E, Torok-Storb B (2006) The stromal component of the marrow microenvironment is not derived from the malignant clone in MDS. Blood 108:772–773
Deeg HJ, Beckham C, Loken MR, Bryant E, Lesnikova M, Shulman HM, Gooley T (2000) Negative regulators of hemopoiesis and stroma function in patients with myelodysplastic syndrome. Leuk Lymphoma 37:405–414
Ximeri M, Galanopoulos A, Klaus M, Parcharidou A, Giannikou K, Psyllaki M, Symeonidis A, Pappa V, Kartasis Z, Liapi D, Hatzimichael E, Kokoris S, Korkolopoulou P, Sambani C, Pontikoglou C, Papadaki HA (2010) Effect of lenalidomide therapy on hematopoiesis of patients with myelodysplastic syndrome associated with chromosome 5q deletion. Haematologica 95:406–414
Bellamy WT, Richter L, Sirjani D, Roxas C, Glinsmann-Gibson B, Frutiger Y, Grogan TM, List AF (2001) Vascular endothelial cell growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood 97:1427–1434
Haase D (2008) Cytogenetic features in myelodysplastic syndromes. Ann Hematol 87:515–526
Patnaik MM, Hanson CA, Hodnefield JM, Knudson R, Van Dyke DL, Tefferi A (2011) Monosomal karyotype in myelodysplastic syndromes, with or without monosomy 7 or 5, is prognostically worse than an otherwise complex karyotype. Leukemia 25:266–270
Heilig CE, Loffler H, Mahlknecht U, Janssen JW, Ho AD, Jauch A, Kramer A (2010) Chromosomal instability correlates with poor outcome in patients with myelodysplastic syndromes irrespectively of the cytogenetic risk group. J Cell Mol Med 14:895–902
Bernasconi P, Klersy C, Boni M, Cavigliano PM, Giardini I, Rocca B, Zappatore R, Dambruoso I, Calvello C, Caresana M, Lazzarino M (2010) Does cytogenetic evolution have any prognostic relevance in myelodysplastic syndromes? A study on 153 patients from a single institution. Ann Hematol 89:545–551
Mallo M, Cervera J, Schanz J, Such E, Garcia-Manero G, Luno E, Steidl C, Espinet B, Vallespi T, Germing U, Blum S, Ohyashiki K, Grau J, Pfeilstocker M, Hernandez JM, Noesslinger T, Giagounidis A, Aul C, Calasanz MJ, Martin ML, Valent P, Collado R, Haferlach C, Fonatsch C, Lubbert M, Stauder R, Hildebrandt B, Krieger O, Pedro C, Arenillas L, Sanz MA, Valencia A, Florensa L, Sanz GF, Haase D, Sole F (2011) Impact of adjunct cytogenetic abnormalities for prognostic stratification in patients with myelodysplastic syndrome and deletion 5q. Leukemia 25:110–120
Gohring G, Giagounidis A, Busche G, Kreipe HH, Zimmermann M, Hellstrom-Lindberg E, Aul C, Schlegelberger B (2010) Patients with del(5q) MDS who fail to achieve sustained erythroid or cytogenetic remission after treatment with lenalidomide have an increased risk for clonal evolution and AML progression. Ann Hematol 89:365–374
Chen G, Zeng W, Miyazato A, Billings E, Maciejewski JP, Kajigaya S, Sloand EM, Young NS (2004) Distinctive gene expression profiles of CD34 cells from patients with myelodysplastic syndrome characterized by specific chromosomal abnormalities. Blood 104:4210–4218
Harada Y, Harada H (2011) Molecular mechanisms that produce secondary MDS/AML by RUNX1/AML1 point mutations. J Cell Biochem 112:425–432
Smith AE, Mohamedali AM, Kulasekararaj A, Lim Z, Gaken J, Lea NC, Przychodzen B, Mian SA, Nasser EE, Shooter C, Westwood NB, Strupp C, Gattermann N, Maciejewski JP, Germing U, Mufti GJ (2010) Next-generation sequencing of the TET2 gene in 355 MDS and CMML patients reveals low-abundance mutant clones with early origins, but indicates no definite prognostic value. Blood 116:3923–3932
Rocquain J, Carbuccia N, Trouplin V, Raynaud S, Murati A, Nezri M, Tadrist Z, Olschwang S, Vey N, Birnbaum D, Gelsi-Boyer V, Mozziconacci MJ (2010) Combined mutations of ASXL1, CBL, FLT3, IDH1, IDH2, JAK2, KRAS, NPM1, NRAS, RUNX1, TET2 and WT1 genes in myelodysplastic syndromes and acute myeloid leukemias. BMC Cancer 10:401
Tamaki H, Ogawa H, Ohyashiki K, Ohyashiki JH, Iwama H, Inoue K, Soma T, Oka Y, Tatekawa T, Oji Y, Tsuboi A, Kim EH, Kawakami M, Fuchigami K, Tomonaga M, Toyama K, Aozasa K, Kishimoto T, Sugiyama H (1999) The Wilms' tumor gene WT1 is a good marker for diagnosis of disease progression of myelodysplastic syndromes. Leukemia 13:393–399
Tamura H, Dan K, Yokose N, Iwakiri R, Ohta M, Sakamaki H, Tohyama K, Kondo A, Hyodo H, Nakamura K, Yamashita T, Elisseeva OA, Oka Y, Oji Y, Sugiyama H, Ogata K (2010) Prognostic significance of WT1 mRNA and anti-WT1 antibody levels in peripheral blood in patients with myelodysplastic syndromes. Leuk Res 34:986–990
Kaneko H, Misawa S, Horiike S, Nakai H, Kashima K (1995) TP53 mutations emerge at early phase of myelodysplastic syndrome and are associated with complex chromosomal abnormalities. Blood 85:2189–2193
Christiansen DH, Andersen MK, Pedersen-Bjergaard J (2001) Mutations with loss of heterozygosity of p53 are common in therapy-related myelodysplasia and acute myeloid leukemia after exposure to alkylating agents and significantly associated with deletion or loss of 5q, a complex karyotype, and a poor prognosis. J Clin Oncol 19:1405–1413
Kitagawa M, Yoshida S, Kuwata T, Tanizawa T, Kamiyama R (1994) p53 expression in myeloid cells of myelodysplastic syndromes. Association with evolution of overt leukemia. Am J Pathol 145:338–344
Iwasaki T, Murakami M, Sugisaki C, Sobue S, Ohashi H, Asano H, Suzuki M, Nakamura S, Ito M, Murate T (2008) Characterization of myelodysplastic syndrome and aplastic anemia by immunostaining of p53 and hemoglobin F and karyotype analysis: differential diagnosis between refractory anemia and aplastic anemia. Pathol Int 58:353–360
Jädersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Göhring G, Hedlund A, Hast R, Schlegelberger B, Porwit A, Hellström-Lindberg E, Mufti GJ. TP53 Mutations in Low-Risk Myelodysplastic Syndromes With del(5q) Predict Disease Progression. J Clin Oncol. 2011 doi:10.1200/JCO.2010.31.8576
Pellagatti A, Cazzola M, Giagounidis A, Perry J, Malcovati L, Della Porta MG, Jadersten M, Killick S, Verma A, Norbury CJ, Hellstrom-Lindberg E, Wainscoat JS, Boultwood J (2010) Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia 24:756–764
Ohyashiki JH, Iwama H, Yahata N, Ando K, Hayashi S, Shay JW, Ohyashiki K (1999) Telomere stability is frequently impaired in high-risk groups of patients with myelodysplastic syndromes. Clin Cancer Res 5:1155–1160
Sieglova Z, Zilovcova S, Cermak J, Rihova H, Brezinova D, Dvorakova R, Markova M, Maaloufova J, Sajdova J, Brezinova J, Zemanova Z, Michalova K (2004) Dynamics of telomere erosion and its association with genome instability in myelodysplastic syndromes (MDS) and acute myelogenous leukemia arising from MDS: a marker of disease prognosis? Leuk Res 28:1013–1021
Lange K, Holm L, Vang NK, Hahn A, Hofmann W, Kreipe H, Schlegelberger B, Gohring G (2010) Telomere shortening and chromosomal instability in myelodysplastic syndromes. Genes Chromosom Cancer 49:260–269
Issa JP (2010) Epigenetic changes in the myelodysplastic syndrome. Hematol Oncol Clin North Am 24:317–330
Esteller M (2002) CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene 21:5427–5440
Jiang Y, Dunbar A, Gondek LP, Mohan S, Rataul M, O’Keefe C, Sekeres M, Saunthararajah Y, Maciejewski JP (2009) Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood 113:1315–1325
Ye Y, McDevitt MA, Guo M, Zhang W, Galm O, Gore SD, Karp JE, Maciejewski JP, Kowalski J, Tsai HL, Gondek LP, Tsai HC, Wang X, Hooker C, Smith BD, Carraway HE, Herman JG (2009) Progressive chromatin repression and promoter methylation of CTNNA1 associated with advanced myeloid malignancies. Cancer Res 69:8482–8490
Zebedee Z, Hara E (2001) Id proteins in cell cycle control and cellular senescence. Oncogene 20:8317–8325
Wang H, Wang XQ, Xu XP, Lin GW (2010) ID4 methylation predicts high risk of leukemic transformation in patients with myelodysplastic syndrome. Leuk Res 34:598–604
Aggerholm A, Holm MS, Guldberg P, Olesen LH, Hokland P (2006) Promoter hypermethylation of p15INK4B, HIC1, CDH1, and ER is frequent in myelodysplastic syndrome and predicts poor prognosis in early-stage patients. Eur J Haematol 76:23–32
Shen L, Kantarjian H, Guo Y, Lin E, Shan J, Huang X, Berry D, Ahmed S, Zhu W, Pierce S, Kondo Y, Oki Y, Jelinek J, Saba H, Estey E, Issa JP (2010) DNA methylation predicts survival and response to therapy in patients with myelodysplastic syndromes. J Clin Oncol 28:605–613
Nikoloski G, Langemeijer SM, Kuiper RP, Knops R, Massop M, Tonnissen ER, van der Heijden A, Scheele TN, Vandenberghe P, de Witte T, van der Reijden BA, Jansen JH (2010) Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet 42:665–667
Chen S, Sang N (2011) Histone deacetylase inhibitors: the epigenetic therapeutics that repress hypoxia-inducible factors. J Biomed Biotechnol 2011:197946
Kubicek S, O'Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K, Kowalski JA, Homon CA, Kelly TA, Jenuwein T (2007) Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell 25:473–481
Kerbauy DB, Deeg HJ (2007) Apoptosis and antiapoptotic mechanisms in the progression of myelodysplastic syndrome. Exp Hematol 35:1739–1746
Parker JE, Mufti GJ, Rasool F, Mijovic A, Devereux S, Pagliuca A (2000) The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood 96:3932–3938
Stifter G, Heiss S, Gastl G, Tzankov A, Stauder R (2005) Over-expression of tumor necrosis factor-alpha in bone marrow biopsies from patients with myelodysplastic syndromes: relationship to anemia and prognosis. Eur J Haematol 75:485–491
Gersuk GM, Beckham C, Loken MR, Kiener P, Anderson JE, Farrand A, Troutt AB, Ledbetter JA, Deeg HJ (1998) A role for tumour necrosis factor-alpha, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. Br J Haematol 103:176–188
Shetty V, Mundle S, Alvi S, Showel M, Broady-Robinson L, Dar S, Borok R, Showel J, Gregory S, Rifkin S, Gezer S, Parcharidou A, Venugopal P, Shah R, Hernandez B, Klein M, Alston D, Robin E, Dominquez C, Raza A (1996) Measurement of apoptosis, proliferation and three cytokines in 46 patients with myelodysplastic syndromes. Leuk Res 20:891–900
Boudard D, Vasselon C, Bertheas MF, Jaubert J, Mounier C, Reynaud J, Viallet A, Chautard S, Guyotat D, Campos L (2002) Expression and prognostic significance of Bcl-2 family proteins in myelodysplastic syndromes. Am J Hematol 70:115–125
Kerbauy DM, Lesnikov V, Abbasi N, Seal S, Scott B, Deeg HJ (2005) NF-kappaB and FLIP in arsenic trioxide (ATO)-induced apoptosis in myelodysplastic syndromes (MDSs). Blood 106:3917–3925
Molldrem JJ, Jiang YZ, Stetler-Stevenson M, Mavroudis D, Hensel N, Barrett AJ (1998) Haematological response of patients with myelodysplastic syndrome to antithymocyte globulin is associated with a loss of lymphocyte-mediated inhibition of CFU-GM and alterations in T-cell receptor Vbeta profiles. Br J Haematol 102:1314–1322
Zou JX, Rollison DE, Boulware D, Chen DT, Sloand EM, Pfannes LV, Goronzy JJ, Bai F, Painter JS, Wei S, Cosgrove D, List AF, Epling-Burnette PK (2009) Altered naive and memory CD4+ T-cell homeostasis and immunosenescence characterize younger patients with myelodysplastic syndrome. Leukemia 23:1288–1296
Kordasti SY, Afzali B, Lim Z, Ingram W, Hayden J, Barber L, Matthews K, Chelliah R, Guinn B, Lombardi G, Farzaneh F, Mufti GJ (2009) IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol 145:64–72
Kotsianidis I, Bouchliou I, Nakou E, Spanoudakis E, Margaritis D, Christophoridou AV, Anastasiades A, Tsigalou C, Bourikas G, Karadimitris A, Tsatalas C (2009) Kinetics, function and bone marrow trafficking of CD4 + CD25 + FOXP3+ regulatory T cells in myelodysplastic syndromes (MDS). Leukemia 23:510–518
Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, Zou J, Ku E, Zhong B, Boulware D, Moscinski L, Wei S, Djeu JY, List AF (2007) Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 109:4816–4824
Thol F, Weissinger EM, Krauter J, Wagner K, Damm F, Wichmann M, Gohring G, Schumann C, Bug G, Ottmann O, Hofmann WK, Schlegelberger B, Ganser A, Heuser M (2010) IDH1 mutations in patients with myelodysplastic syndromes are associated with an unfavorable prognosis. Haematologica 95:1668–1674
Boultwood J, Perry J, Pellagatti A, Fernandez-Mercado M, Fernandez-Santamaria C, Calasanz MJ, Larrayoz MJ, Garcia-Delgado M, Giagounidis A, Malcovati L, Della Porta MG, Jadersten M, Killick S, Hellstrom-Lindberg E, Cazzola M, Wainscoat JS (2010) Frequent mutation of the polycomb-associated gene ASXL1 in the myelodysplastic syndromes and in acute myeloid leukemia. Leukemia 24:1062–1065
Chen CY, Lin LI, Tang JL, Ko BS, Tsay W, Chou WC, Yao M, Wu SJ, Tseng MH, Tien HF (2007) RUNX1 gene mutation in primary myelodysplastic syndrome—the mutation can be detected early at diagnosis or acquired during disease progression and is associated with poor outcome. Br J Haematol 139:405–414
Georgiou G, Karali V, Zouvelou C, Kyriakou E, Dimou M, Chrisochoou S, Greka P, Dufexis D, Vervesou E, Dimitriadou E, Efthymiou A, Petrikkos L, Dima K, Lilakos K, Panayiotidis P (2006) Serial determination of FLT3 mutations in myelodysplastic syndrome patients at diagnosis, follow up or acute myeloid leukaemia transformation: incidence and their prognostic significance. Br J Haematol 134:302–306
Bains A, Luthra R, Medeiros LJ, Zuo Z (2011) FLT3 and NPM1 mutations in myelodysplastic syndromes: frequency and potential value for predicting progression to acute myeloid leukemia. Am J Clin Pathol 135:62–69
Paquette RL, Landaw EM, Pierre RV, Kahan J, Lubbert M, Lazcano O, Isaac G, McCormick F, Koeffler HP (1993) N-ras mutations are associated with poor prognosis and increased risk of leukemia in myelodysplastic syndrome. Blood 82:590–599
Bacher U, Haferlach T, Kern W, Haferlach C, Schnittger S (2007) A comparative study of molecular mutations in 381 patients with myelodysplastic syndrome and in 4130 patients with acute myeloid leukemia. Haematologica 92:744–752
Lorenzo F, Nishii K, Monma F, Kuwagata S, Usui E, Shiku H (2006) Mutational analysis of the KIT gene in myelodysplastic syndrome (MDS) and MDS-derived leukemia. Leuk Res 30:1235–1239
Russell M, List A, Greenberg P, Woodward S, Glinsmann B, Parganas E, Ihle J, Taetle R (1994) Expression of EVI1 in myelodysplastic syndromes and other hematologic malignancies without 3q26 translocations. Blood 84:1243–1248
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Based on a lecture delivered at the Xth EBMWG International Course in Bone Marrow Pathology, 14–16th May, 2011, London, UK
Rights and permissions
About this article
Cite this article
Porwit, A., Saft, L. The AML–MDS interface—leukemic transformation in myelodysplastic syndromes. J Hematopathol 4, 69–79 (2011). https://doi.org/10.1007/s12308-011-0088-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-011-0088-6