Abstract
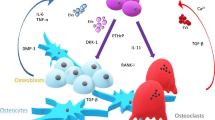
Breast cancer is the most prevalent cancer among females worldwide. It has long been known that cancers preferentially metastasize to particular organs, and bone metastases occur in ∼70% of patients with advanced breast cancer. Breast cancer bone metastases are predominantly osteolytic and accompanied by bone destruction, bone fractures, pain, and hypercalcemia, causing severe morbidity and hospitalization. In the bone matrix, transforming growth factor-β (TGF-β) is one of the most abundant growth factors, which is released in active form upon tumor-induced osteoclastic bone resorption. TGF-β, in turn, stimulates bone metastatic cells to secrete factors that further drive osteolytic destruction of the bone adjacent to the tumor, categorizing TGF-β as a crucial factor responsible for driving the feed-forward vicious cycle of cancer growth in bone. Moreover, TGF-β activates epithelial-to-mesenchymal transition, increases tumor cell invasiveness and angiogenesis and induces immunosuppression. Blocking the TGF-β signaling pathway to interrupt this vicious cycle between breast cancer and bone offers a promising target for therapeutic intervention to decrease skeletal metastasis. This review will describe the role of TGF-β in breast cancer and bone metastasis, and pre-clinical and clinical data will be evaluated for the potential use of TGF-β inhibitors in clinical practice to treat breast cancer bone metastases.



Similar content being viewed by others
Abbreviations
- ALK:
-
Activin receptor-like kinase
- ASO:
-
Antisense oligonucleotides
- BMP:
-
Bone morphogenetic protein
- BSP:
-
Bone sialoprotein
- CAT:
-
Cambridge antibody technology
- CSC:
-
Cancer stem cell
- DN:
-
Dominant negative
- EMT:
-
Epithelial-to-mesenchymal transition
- GM-CSF:
-
Granulocyte macrophage colony stimulating factor (a.k.a. CSF2)
- Hfg:
-
Halofuginone
- HIF:
-
Hypoxia inducible factor
- HMEC:
-
Human mammary epithelial cells
- i.v.:
-
Intravenous
- i.p.:
-
Intraperitoneal
- IGF:
-
Insulin growth factor
- IL:
-
Interleukin
- JNK:
-
JunN-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MET:
-
Mesenchymal-to-epithelial transition
- MMTV:
-
Mouse mammary tumor virus
- OPG:
-
Osteoprotegerin
- OPN:
-
Osteopontin
- PDGF:
-
Platelet-derived growth factor
- PTHrP:
-
Parathyroid hormone-related protein
- R-Smads:
-
Receptor-regulated Smads
- RANK:
-
Receptor activator of nuclear factor κB
- RANKL:
-
Receptor activator of nuclear factor κB ligand
- s.c.:
-
Subcutaneous
- SDF-1:
-
Stromal derived growth factor-1
- TβRI:
-
Transforming growth factor-β type I receptor (a.k.a ALK5)
- TβRII:
-
Transforming growth factor-β type II receptor
- TGF-β:
-
Transforming growth factor-β
- VEGF:
-
Vascular endothelial growth factor
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Canc J Clin 61(2):69–90. doi:10.3322/caac.20107
Buijs JT, van der Pluijm G (2009) Osteotropic cancers: from primary tumor to bone. Canc Lett 273(2):177–193. doi:10.1016/j.canlet.2008.05.044
Coleman RE (1997) Skeletal complications of malignancy. Cancer 80(8 Suppl):1588–1594. doi:10.1002/(SICI)1097-0142(19971015)80:8
Mundy GR (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Canc 2(8):584–593. doi:10.1038/nrc867
Roodman GD (2004) Mechanisms of bone metastasis. New Engl J Med 350(16):1655–1664. doi:10.1056/NEJMra030831
Korpal M, Yan J, Lu X, Xu S, Lerit DA, Kang Y (2009) Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat Med 15(8):960–966. doi:10.1038/nm.1943
Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J (2003) A multigenic program mediating breast cancer metastasis to bone. Canc Cell 3(6):537–549
Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA (1999) TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Investig 103(2):197–206. doi:10.1172/JCI3523
Yingling JM, Blanchard KL, Sawyer JS (2004) Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov 3(12):1011–1022. doi:10.1038/nrd1580
Massague J, Blain SW, Lo RS (2000) TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103(2):295–309
Blobe GC, Schiemann WP, Lodish HF (2000) Role of transforming growth factor beta in human disease. New Engl J Med 342(18):1350–1358
ten Dijke P, Arthur HM (2007) Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol 8(11):857–869. doi:10.1038/nrm2262
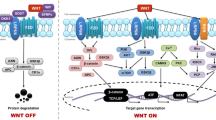
Massague J (2000) How cells read TGF-beta signals. Nat Rev Mol Cell Biol 1(3):169–178. doi:10.1038/35043051
Feng XH, Derynck R (2005) Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 21:659–693. doi:10.1146/annurev.cellbio.21.022404.142018
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425(6958):577–584. doi:10.1038/nature02006
Wu MY, Hill CS (2009) Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell 16(3):329–343. doi:10.1016/j.devcel.2009.02.012
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113(6):685–700
Ikushima H, Komuro A, Isogaya K, Shinozaki M, Hellman U, Miyazawa K, Miyazono K (2008) An Id-like molecule, HHM, is a synexpression group-restricted regulator of TGF-beta signalling. EMBO J 27(22):2955–2965. doi:10.1038/emboj.2008.218
Ikushima H, Miyazono K (2010) TGFbeta signalling: a complex web in cancer progression. Nat Rev Canc 10(6):415–424. doi:10.1038/nrc2853
Daly AC, Randall RA, Hill CS (2008) Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol 28(22):6889–6902. doi:10.1128/MCB.01192-08
Liu IM, Schilling SH, Knouse KA, Choy L, Derynck R, Wang XF (2009) TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. EMBO J 28(2):88–98. doi:10.1038/emboj.2008.266
Pardali E, Goumans MJ, Ten Dijke P (2010) Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol 20(9):556–567. doi:10.1016/j.tcb.2010.06.006
Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P (2003) Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell 12(4):817–828
Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P (2002) Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 21(7):1743–1753. doi:10.1093/emboj/21.7.1743
Siegel PM, Shu W, Massague J (2003) Mad upregulation and Id2 repression accompany transforming growth factor (TGF)-beta-mediated epithelial cell growth suppression. J Biol Chem 278(37):35444–35450. doi:10.1074/jbc.M301413200
Derynck R, Akhurst RJ (2007) Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol 9(9):1000–1004. doi:10.1038/ncb434
Massague J, Chen YG (2000) Controlling TGF-beta signaling. Gene Dev 14(6):627–644
Calonge MJ, Massague J (1999) Smad4/DPC4 silencing and hyperactive Ras jointly disrupt transforming growth factor-beta antiproliferative responses in colon cancer cells. J Biol Chem 274(47):33637–33643
Massague J (2008) TGFbeta in Cancer. Cell 134(2):215–230. doi:10.1016/j.cell.2008.07.001
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890. doi:10.1016/j.cell.2009.11.007
Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J, Patel SC, Khozin S, Liu ZY, Green J, Anver MR, Merlino G, Wakefield LM (2002) Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Investig 109(12):1607–1615. doi:10.1172/JCI15333
Gorska AE, Jensen RA, Shyr Y, Aakre ME, Bhowmick NA, Moses HL (2003) Transgenic mice expressing a dominant-negative mutant type II transforming growth factor-beta receptor exhibit impaired mammary development and enhanced mammary tumor formation. Am J Pathol 163(4):1539–1549
Lenferink AE, Magoon J, Pepin MC, Guimond A, O’Connor-McCourt MD (2003) Expression of TGF-beta type II receptor antisense RNA impairs TGF-beta signaling in vitro and promotes mammary gland differentiation in vivo. Int J Canc 107(6):919–928. doi:10.1002/ijc.11494
Muraoka RS, Koh Y, Roebuck LR, Sanders ME, Brantley-Sieders D, Gorska AE, Moses HL, Arteaga CL (2003) Increased malignancy of Neu-induced mammary tumors overexpressing active transforming growth factor beta1. Mol Cell Biol 23(23):8691–8703
Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J (2003) Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci USA 100(14):8430–8435. doi:10.1073/pnas.0932636100
Forrester E, Chytil A, Bierie B, Aakre M, Gorska AE, Sharif-Afshar AR, Muller WJ, Moses HL (2005) Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Canc Res 65(6):2296–2302. doi:10.1158/0008-5472.CAN-04-3272
Muraoka-Cook RS, Shin I, Yi JY, Easterly E, Barcellos-Hoff MH, Yingling JM, Zent R, Arteaga CL (2006) Activated type I TGFbeta receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene 25(24):3408–3423. doi:10.1038/sj.onc.1208964
Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA (1992) Immunohistochemical staining for transforming growth factor beta 1 associates with disease progression in human breast cancer. Canc Res 52(24):6949–6952
Tan AR, Alexe G, Reiss M (2009) Transforming growth factor-beta signaling: emerging stem cell target in metastatic breast cancer? Breast Canc Res Treat 115(3):453–495. doi:10.1007/s10549-008-0184-1
Travers MT, Barrett-Lee PJ, Berger U, Luqmani YA, Gazet JC, Powles TJ, Coombes RC (1988) Growth factor expression in normal, benign, and malignant breast tissue. Br Med J (Clin Res Ed) 296(6637):1621–1624
de Jong JS, van Diest PJ, van der Valk P, Baak JP (1998) Expression of growth factors, growth-inhibiting factors, and their receptors in invasive breast cancer. II: Correlations with proliferation and angiogenesis. J Pathol 184(1):53–57. doi:10.1002/(SICI)1096-9896(199801)184:1
Grau AM, Wen W, Ramroopsingh DS, Gao YT, Zi J, Cai Q, Shu XO, Zheng W (2008) Circulating transforming growth factor-beta-1 and breast cancer prognosis: results from the Shanghai Breast Cancer Study. Breast Canc Res Treat 112(2):335–341. doi:10.1007/s10549-007-9845-8
Ivanovic V, Todorovic-Rakovic N, Demajo M, Neskovic-Konstantinovic Z, Subota V, Ivanisevic-Milovanovic O, Nikolic-Vukosavljevic D (2003) Elevated plasma levels of transforming growth factor-beta 1 (TGF-beta 1) in patients with advanced breast cancer: association with disease progression. Eur J Canc 39(4):454–461
Kong FM, Anscher MS, Murase T, Abbott BD, Iglehart JD, Jirtle RL (1995) Elevated plasma transforming growth factor-beta 1 levels in breast cancer patients decrease after surgical removal of the tumor. Ann Surg 222(2):155–162
Sheen-Chen SM, Chen HS, Sheen CW, Eng HL, Chen WJ (2001) Serum levels of transforming growth factor beta1 in patients with breast cancer. Arch Surg 136(8):937–940
Baselga J, Rothenberg ML, Tabernero J, Seoane J, Daly T, Cleverly A, Berry B, Rhoades SK, Ray CA, Fill J, Farrington DL, Wallace LA, Yingling JM, Lahn M, Arteaga C, Carducci M (2008) TGF-beta signalling-related markers in cancer patients with bone metastasis. Biomarkers 13(2):217–236. doi:10.1080/13547500701676019
Buck MB, Fritz P, Dippon J, Zugmaier G, Knabbe C (2004) Prognostic significance of transforming growth factor beta receptor II in estrogen receptor-negative breast cancer patients. Clin Canc Res 10(2):491–498
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988. doi:10.1073/pnas.0530291100
Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ (2005) Prospective identification of tumorigenic prostate cancer stem cells. Canc Res 65(23):10946–10951. doi:10.1158/0008-5472.CAN-05-2018
O’Brien CA, Pollett A, Gallinger S, Dick JE (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445(7123):106–110. doi:10.1038/nature05372
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414(6859):105–111. doi:10.1038/35102167
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445(7123):111–115. doi:10.1038/nature05384
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432(7015):396–401. doi:10.1038/nature03128
van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Lippitt JM, Guzman-Ramirez N, Hamdy FC, Eaton CL, Thalmann GN, Cecchini MG, Pelger RC, van der Pluijm G (2010) High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Canc Res 70(12):5163–5173. doi:10.1158/0008-5472.CAN-09-3806
Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS et al (2007) Molecular definition of breast tumor heterogeneity. Canc Cell 11(3):259–273. doi:10.1016/j.ccr.2007.01.013
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715. doi:10.1016/j.cell.2008.03.027
Seyedin SM, Thomas TC, Thompson AY, Rosen DM, Piez KA (1985) Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc Natl Acad Sci USA 82(8):2267–2271
Seyedin SM, Thompson AY, Bentz H, Rosen DM, McPherson JM, Conti A, Siegel NR, Galluppi GR, Piez KA (1986) Cartilage-inducing factor-A. Apparent identity to transforming growth factor-beta. J Biol Chem 261(13):5693–5695
Janssens K, ten Dijke P, Janssens S, Van Hul W (2005) Transforming growth factor-beta1 to the bone. Endocr Rev 26(6):743–774. doi:10.1210/er.2004-0001
Iqbal J, Sun L, Zaidi M (2009) Coupling bone degradation to formation. Nat Med 15(7):729–731. doi:10.1038/nm0709-729
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X (2009) TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 15(7):757–765. doi:10.1038/nm.1979
Wu X, Pang L, Lei W, Lu W, Li J, Li Z, Frassica FJ, Chen X, Wan M, Cao X (2010) Inhibition of sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell. doi:10.1016/j.stem.2010.09.012
Canalis E (2009) Growth factor control of bone mass. J Cell Biochem 108(4):769–777. doi:10.1002/jcb.22322
Alliston T, Choy L, Ducy P, Karsenty G, Derynck R (2001) TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J 20(9):2254–2272. doi:10.1093/emboj/20.9.2254
Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K (2004) Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J 23(3):552–563. doi:10.1038/sj.emboj.7600067
Karsdal MA, Andersen TA, Bonewald L, Christiansen C (2004) Matrix metalloproteinases (MMPs) safeguard osteoblasts from apoptosis during transdifferentiation into osteocytes: MT1-MMP maintains osteocyte viability. DNA Cell Biol 23(3):155–165. doi:10.1089/104454904322964751
Quinn JM, Itoh K, Udagawa N, Hausler K, Yasuda H, Shima N, Mizuno A, Higashio K, Takahashi N, Suda T, Martin TJ, Gillespie MT (2001) Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. J Bone Miner Res 16(10):1787–1794. doi:10.1359/jbmr.2001.16.10.1787
Sells Galvin RJ, Gatlin CL, Horn JW, Fuson TR (1999) TGF-beta enhances osteoclast differentiation in hematopoietic cell cultures stimulated with RANKL and M-CSF. Biochem Biophys Res Commun 265(1):233–239. doi:10.1006/bbrc.1999.1632
Balooch G, Balooch M, Nalla RK, Schilling S, Filvaroff EH, Marshall GW, Marshall SJ, Ritchie RO, Derynck R, Alliston T (2005) TGF-beta regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci USA 102(52):18813–18818. doi:10.1073/pnas.0507417102
Erlebacher A, Derynck R (1996) Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype. J Cell Biol 132(1–2):195–210
Mohammad KS, Chen CG, Balooch G, Stebbins E, McKenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH, Ionova-Martin SS, Bracey JW, Hogue WR, Wong DH, Ritchie RO, Suva LJ, Derynck R, Guise TA, Alliston T (2009) Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS One 4(4):e5275. doi:10.1371/journal.pone.0005275
DaCosta BS, Major C, Laping NJ, Roberts AB (2004) SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol 65(3):744–752. doi:10.1124/mol.65.3.744
Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62(1):65–74
Petersen M, Thorikay M, Deckers M, van Dinther M, Grygielko ET, Gellibert F, de Gouville AC, Huet S, ten Dijke P, Laping NJ (2008) Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int 73(6):705–715. doi:10.1038/sj.ki.5002717
Tojo M, Hamashima Y, Hanyu A, Kajimoto T, Saitoh M, Miyazono K, Node M, Imamura T (2005) The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Canc Sci 96(11):791–800. doi:10.1111/j.1349-7006.2005.00103.x
Eijken M, Swagemakers S, Koedam M, Steenbergen C, Derkx P, Uitterlinden AG, van der Spek PJ, Visser JA, de Jong FH, Pols HA, van Leeuwen JP (2007) The activin A-follistatin system: potent regulator of human extracellular matrix mineralization. FASEB J 21(11):2949–2960. doi:10.1096/fj.07-8080com
Pearsall RS, Canalis E, Cornwall-Brady M, Underwood KW, Haigis B, Ucran J, Kumar R, Pobre E, Grinberg A, Werner ED, Glatt V, Stadmeyer L, Smith D, Seehra J, Bouxsein ML (2008) A soluble activin type IIA receptor induces bone formation and improves skeletal integrity. Proc Natl Acad Sci USA 105(19):7082–7087. doi:10.1073/pnas.0711263105
Edwards JR, Nyman JS, Lwin ST, Moore MM, Esparza J, O’Quinn EC, Hart AJ, Biswas S, Patil CA, Lonning S, Mahadevan-Jansen A, Mundy GR (2010) Inhibition of TGF-beta signaling by 1D11 antibody treatment increases bone mass and quality in vivo. J Bone Miner Res. doi:10.1002/jbmr.139
Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA, Grubbs BG, Dallas M, Cui Y, Guise TA (2002) Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem 277(27):24571–24578. doi:10.1074/jbc.M202561200
Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR (1996) Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Investig 98(7):1544–1549. doi:10.1172/JCI118947
Clines GA, Guise TA (2005) Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocr Relat Canc 12(3):549–583. doi:10.1677/erc.1.00543
Mundy GR, Edwards JR (2008) PTH-related peptide (PTHrP) in hypercalcemia. J Am Soc Nephrol 19(4):672–675. doi:10.1681/ASN.2007090981
Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, Gillespie MT (1999) Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology 140(10):4451–4458
Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, Bennett RC, Martin TJ (1991) Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Canc Res 51(11):3059–3061
Southby J, Kissin MW, Danks JA, Hayman JA, Moseley JM, Henderson MA, Bennett RC, Martin TJ (1990) Immunohistochemical localization of parathyroid hormone-related protein in human breast cancer. Canc Res 50(23):7710–7716
Bundred NJ, Walker RA, Ratcliffe WA, Warwick J, Morrison JM, Ratcliffe JG (1992) Parathyroid hormone related protein and skeletal morbidity in breast cancer. Eur J Canc 28(2–3):690–692
Kissin MW, Henderson MA, Danks JA, Hayman JA, Bennett RC, Martin TJ (1993) Parathyroid hormone related protein in breast cancers of widely varying prognosis. Eur J Surg Oncol 19(2):134–142
Henderson M, Danks J, Moseley J, Slavin J, Harris T, McKinlay M, Hopper J, Martin T (2001) Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. J Natl Canc Inst 93(3):234–237
Henderson MA, Danks JA, Slavin JL, Byrnes GB, Choong PF, Spillane JB, Hopper JL, Martin TJ (2006) Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Canc Res 66(4):2250–2256. doi:10.1158/0008-5472.CAN-05-2814
Kohno N, Kitazawa S, Sakoda Y, Kanbara Y, Furuya Y, Ohashi O, Kitazawa R (1994) Parathyroid hormone-related protein in breast cancer tissues: relationship between primary and metastatic sites. Breast Canc 1(1):43–49
Guise TA (2009) Breaking down bone: new insight into site-specific mechanisms of breast cancer osteolysis mediated by metalloproteinases. Gene Dev 23(18):2117–2123. doi:10.1101/gad.1854909
Lu X, Wang Q, Hu G, Van Poznak C, Fleisher M, Reiss M, Massague J, Kang Y (2009) ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Gene Dev 23(16):1882–1894. doi:10.1101/gad.1824809
Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, Peng XH, Chirgwin JM, Guise TA (2009) Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One 4(9):e6896. doi:10.1371/journal.pone.0006896
Kang Y, Massague J (2004) Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118(3):277–279. doi:10.1016/j.cell.2004.07.011
Deckers M, van Dinther M, Buijs J, Que I, Lowik C, van der Pluijm G, ten Dijke P (2006) The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Canc Res 66(4):2202–2209. doi:10.1158/0008-5472.CAN-05-3560
Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL, Massague J (2005) Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA 102(39):13909–13914. doi:10.1073/pnas.0506517102
Sethi N, Dai X, Winter CG, Kang Y (2011) Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Canc Cell 19(2):192–205. doi:10.1016/j.ccr.2010.12.022
Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, ten Dijke P, van der Pluijm G (2007) TGF-beta and BMP7 interactions in tumour progression and bone metastasis. Clin Exp Metastasis 24(8):609–617. doi:10.1007/s10585-007-9118-2
Serganova I, Moroz E, Vider J, Gogiberidze G, Moroz M, Pillarsetty N, Doubrovin M, Minn A, Thaler HT, Massague J, Gelovani J, Blasberg R (2009) Multimodality imaging of TGFbeta signaling in breast cancer metastases. FASEB J 23(8):2662–2672. doi:10.1096/fj.08-126920
Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS (2008) Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Canc 8(3):212–226. doi:10.1038/nrc2345
Bellahcene A, Kroll M, Liebens F, Castronovo V (1996) Bone sialoprotein expression in primary human breast cancer is associated with bone metastases development. J Bone Miner Res 11(5):665–670. doi:10.1002/jbmr.5650110514
Hotte SJ, Winquist EW, Stitt L, Wilson SM, Chambers AF (2002) Plasma osteopontin: associations with survival and metastasis to bone in men with hormone-refractory prostate carcinoma. Cancer 95(3):506–512. doi:10.1002/cncr.10709
Zhang L, Hou X, Lu S, Rao H, Hou J, Luo R, Huang H, Zhao H, Jian H, Chen Z, Liao M, Wang X (2010) Predictive significance of bone sialoprotein and osteopontin for bone metastases in resected Chinese non-small-cell lung cancer patients: a large cohort retrospective study. Lung Canc 67(1):114–119. doi:10.1016/j.lungcan.2009.03.017
Brown JM, Wilson WR (2004) Exploiting tumour hypoxia in cancer treatment. Nat Rev Canc 4(6):437–447. doi:10.1038/nrc1367
Hiraga T, Kizaka-Kondoh S, Hirota K, Hiraoka M, Yoneda T (2007) Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Canc Res 67(9):4157–4163. doi:10.1158/0008-5472.CAN-06-2355
McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM (2006) Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem 281(34):24171–24181. doi:10.1074/jbc.M604507200
Lu X, Yan CH, Yuan M, Wei Y, Hu G, Kang Y (2010) In vivo dynamics and distinct functions of hypoxia in primary tumor growth and organotropic metastasis of breast cancer. Canc Res 70(10):3905–3914. doi:10.1158/0008-5472.CAN-09-3739[doi]
Buijs JT, Kuijpers CC, van der Pluijm G (2010) Targeted therapy options for treatment of bone metastases; beyond bisphosphonates. Curr Pharm Des 16(27):13
Kelly RJ, Morris JC (2010) Transforming growth factor-beta: a target for cancer therapy. J Immunotoxicol 7(1):15–26. doi:10.3109/15476910903389920
Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals PJ, Koteliansky V, Arteaga CL (2002) Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Investig 109(12):1551–1559. doi:10.1172/JCI15234
Muraoka-Cook RS, Kurokawa H, Koh Y, Forbes JT, Roebuck LR, Barcellos-Hoff MH, Moody SE, Chodosh LA, Arteaga CL (2004) Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Canc Res 64(24):9002–9011. doi:10.1158/0008-5472.CAN-04-2111
Arteaga CL, Carty-Dugger T, Moses HL, Hurd SD, Pietenpol JA (1993) Transforming growth factor beta 1 can induce estrogen-independent tumorigenicity of human breast cancer cells in athymic mice. Cell Growth Differ 4(3):193–201
Arteaga CL, Hurd SD, Winnier AR, Johnson MD, Fendly BM, Forbes JT (1993) Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J Clin Investig 92(6):2569–2576. doi:10.1172/JCI116871
Nam JS, Suchar AM, Kang MJ, Stuelten CH, Tang B, Michalowska AM, Fisher LW, Fedarko NS, Jain A, Pinkas J, Lonning S, Wakefield LM (2006) Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor beta in a mouse model of breast cancer. Canc Res 66(12):6327–6335. doi:10.1158/0008-5472.CAN-06-0068
Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S, Berzofsky JA, Wakefield LM (2008) Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Canc Res 68(10):3915–3923. doi:10.1158/0008-5472.CAN-08-0206
Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, Kohn E, Tang B, Sabzevari H, Anver MR, Lawrence S, Danielpour D, Lonning S, Berzofsky JA, Wakefield LM (2008) An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Canc Res 68(10):3835–3843. doi:10.1158/0008-5472.CAN-08-0215
Biswas S, Wilburn C, Munoz SA, Sterling JA, Lonning S, Mundy GR (2008) Monoclonal antibody to transforming growth factor b inhibits tumor burden and osteolysis in a pre-clinical model of bone metastasis. J Bone Miner Res 23 (SA187)
Mead AL, Wong TT, Cordeiro MF, Anderson IK, Khaw PT (2003) Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci 44(8):3394–3401
Thompson JE, Vaughan TJ, Williams AJ, Wilton J, Johnson KS, Bacon L, Green JA, Field R, Ruddock S, Martins M, Pope AR, Tempest PR, Jackson RH (1999) A fully human antibody neutralising biologically active human TGFbeta2 for use in therapy. J Immunol Meth 227(1–2):17–29
Cordeiro MF, Gay JA, Khaw PT (1999) Human anti-transforming growth factor-beta2 antibody: a new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci 40(10):2225–2234
Genzyme (2007) Safety study of GC1008 in patients with focal segmental glomerulosclerosis (FSGS) of single doses of GC1008 in patients with treatment resistant idiopathic FSGS. National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00464321. Accessed 2010 Aug 23
Genzyme (2006) Safety and efficacy study of GC1008 to treat renal cell carcinoma or malignant melanoma. National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00356460. Accessed 2010 Aug 23
Jersey CIoN (2008) Effects of monoclonal antibody GC1008 in blood samples from patients with unresectable locally advanced or metastatic kidney cancer or malignant melanoma treated on clinical trial NCI-06-C-0200. National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00899444. Accessed 2010 Aug 23
Morris JC, Shapiro GI, Tan AR, Lawrence DP, Olencki TE, Dezube BJ, Hsu FJ, Reiss M, Berzofsky JA (2008) Phase I/II Study of GC1008: a human anti-transforming growth factor-beta (TGF-β) monoclonal antibody (MAb) in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC). J Clin Oncol 26(Suppl):9028
Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, Rota S, Remuzzi G, Rump LC, Sellin LK, Heaton JP, Streisand JB, Hard ML, Ledbetter SR, Vincenti F (2011) A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int 79(11):1236–1243. doi:10.1038/ki.2011.33
Genzyme (2005) Study of GC1008 in patients with idiopathic pulmonary fibrosis (IPF). National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00125385. Accessed 2010 Aug 24
Mancuso P, Shalinsky DR, Calleri A, Quarna J, Antoniotti P, Jilani I, Hu-Lowe D, Jiang X, Gallo-Stampino C, Bertolini F (2009) Evaluation of ALK-1 expression in circulating endothelial cells (CECs) as an exploratory biomarker for PF-03446962 undergoing phase I trial in cancer patients. J Clin Oncol 27:15s:abstr 3573
Pfizer (2007) A first in patient, study of investigational drug PF-03446962 in patients with advanced solid tumors. National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00557856. Accessed 2010 Aug 23
Bandyopadhyay A, Zhu Y, Cibull ML, Bao L, Chen C, Sun L (1999) A soluble transforming growth factor beta type III receptor suppresses tumorigenicity and metastasis of human breast cancer MDA-MB-231 cells. Canc Res 59(19):5041–5046
Bandyopadhyay A, Lopez-Casillas F, Malik SN, Montiel JL, Mendoza V, Yang J, Sun LZ (2002) Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Canc Res 62(16):4690–4695
Serrati S, Margheri F, Pucci M, Cantelmo AR, Cammarota R, Dotor J, Borras-Cuesta F, Fibbi G, Albini A, Del Rosso M (2009) TGFbeta1 antagonistic peptides inhibit TGFbeta1-dependent angiogenesis. Biochem Pharmacol 77(5):813–825. doi:10.1016/j.bcp.2008.10.036
ISDIN (2007) Efficacy and safety study of p144 to treat skin fibrosis in systemic sclerosis. National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00574613. Accessed 2010 Aug 23
Bennett CF, Swayze EE (2010) RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol 50:259–293. doi:10.1146/annurev.pharmtox.010909.105654
Crooke ST (2004) Progress in antisense technology. Annu Rev Med 55:61–95. doi:10.1146/annurev.med.55.091902.104408
Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A, Brawanski A, Proescholdt M, Schlaier J, Buchroithner J, Pichler J, Wurm G, Mehdorn M, Strege R, Schuierer G, Villarrubia V, Fellner F, Jansen O, Straube T, Nohria V et al (2007) Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides 17(2):201–212. doi:10.1089/oli.2006.0053
Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, Balasubramaniam A, Nair S, Oliushine V, Parfenov V, Poverennova I, Zaaroor M, Jachimczak P, Ludwig S, Schmaus S, Heinrichs H, Schlingensiepen KH (2011) Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol 13(1):132–142. doi:10.1093/neuonc/noq142
Antisense Pharma (2008) Efficacy and safety of AP 12009 in patients with recurrent or refractory anaplastic astrocytoma (SAPPHIRE). National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00761280. Accessed 2010 Aug 23
Antisense Pharma (2009) Safety and tolerability of AP 12009, administered I.V. in patients with advanced tumors known to overproduce TGF-beta-2. National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00844064. Accessed 2010 Aug 23
Schlingensiepen KH (2004) The TGF-β1 antisense oligonucleotide AP 11014 for the treatment of non-small cell lung, colorectal and prostate cancer: preclinical studies. Abstract #3132. In: Am Soc Clin Oncol Ann Meet
Fakhrai H, Dorigo O, Shawler DL, Lin H, Mercola D, Black KL, Royston I, Sobol RE (1996) Eradication of established intracranial rat gliomas by transforming growth factor beta antisense gene therapy. Proc Natl Acad Sci USA 93(7):2909–2914
Fakhrai H, Mantil JC, Liu L, Nicholson GL, Murphy-Satter CS, Ruppert J, Shawler DL (2006) Phase I clinical trial of a TGF-beta antisense-modified tumor cell vaccine in patients with advanced glioma. Canc Gene Ther 13(12):1052–1060. doi:10.1038/sj.cgt.7700975
Gradalis I (2008) Phase I trial of TGFB2-antisense-GMCSF gene modified autologous tumor cell (TAG) vaccine for advanced cancer (Auto TAG). National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00684294. Accessed 2010 Aug 23
Byfield SD, Roberts AB (2004) Lateral signaling enhances TGF-beta response complexity. Trends Cell Biol 14(3):107–111
Fu K, Corbley MJ, Sun L, Friedman JE, Shan F, Papadatos JL, Costa D, Lutterodt F, Sweigard H, Bowes S, Choi M, Boriack-Sjodin PA, Arduini RM, Sun D, Newman MN, Zhang X, Mead JN, Chuaqui CE, Cheung HK, Cornebise M et al (2008) SM16, an orally active TGF-beta type I receptor inhibitor prevents myofibroblast induction and vascular fibrosis in the rat carotid injury model. Arterioscler Thromb Vasc Biol 28(4):665–671. doi:10.1161/ATVBAHA.107.158030
Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, Gaster L, Callahan JF, Olson BA (2002) Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol 62(1):58–64
Ehata S, Hanyu A, Fujime M, Katsuno Y, Fukunaga E, Goto K, Ishikawa Y, Nomura K, Yokoo H, Shimizu T, Ogata E, Miyazono K, Shimizu K, Imamura T (2007) Ki26894, a novel transforming growth factor-beta type I receptor kinase inhibitor, inhibits in vitro invasion and in vivo bone metastasis of a human breast cancer cell line. Canc Sci 98(1):127–133. doi:10.1111/j.1349-7006.2006.00357.x
Bandyopadhyay A, Agyin JK, Wang L, Tang Y, Lei X, Story BM, Cornell JE, Pollock BH, Mundy GR, Sun LZ (2006) Inhibition of pulmonary and skeletal metastasis by a transforming growth factor-beta type I receptor kinase inhibitor. Canc Res 66(13):6714–6721. doi:10.1158/0008-5472.CAN-05-3565
Ge R, Rajeev V, Ray P, Lattime E, Rittling S, Medicherla S, Protter A, Murphy A, Chakravarty J, Dugar S, Schreiner G, Barnard N, Reiss M (2006) Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clin Canc Res 12(14 Pt 1):4315–4330. doi:10.1158/1078-0432.CCR-06-0162
Subramanian G, Schwarz RE, Higgins L, McEnroe G, Chakravarty S, Dugar S, Reiss M (2004) Targeting endogenous transforming growth factor beta receptor signaling in SMAD4-deficient human pancreatic carcinoma cells inhibits their invasive phenotype1. Canc Res 64(15):5200–5211. doi:10.1158/0008-5472.CAN-04-0018
Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R, Mangadu R, Liu YW, Platten M, Herrlinger U, Murphy A, Wong DH, Wick W, Higgins LS, Weller M (2004) SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Canc Res 64(21):7954–7961. doi:10.1158/0008-5472.CAN-04-1013
Gaspar NJ, Li L, Kapoun AM, Medicherla S, Reddy M, Li G, O’Young G, Quon D, Henson M, Damm DL, Muiru GT, Murphy A, Higgins LS, Chakravarty S, Wong DH (2007) Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Mol Pharmacol 72(1):152–161. doi:10.1124/mol.106.029025
Fournier PJ, Mohammad KS, McKenna CR, Peng X, Chirgwin JM, Guise TA (2008) TGF-beta blockade inhibits osteolytic metastases but not osteoblastic prostate cancer metastases. #SA274. In: American Society for Bone and Mineral Research 30th annual meeting, Montreal, QC, Canada
Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM (2006) Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Canc Res 12(20 Pt 2):6213s–6216s. doi:10.1158/1078-0432.CCR-06-1007
Zhang B, Halder SK, Zhang S, Datta PK (2009) Targeting transforming growth factor-beta signaling in liver metastasis of colon cancer. Canc Lett 277(1):114–120. doi:10.1016/j.canlet.2008.11.035
Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, Abbruzzese JL, Chiao PJ (2008) LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Canc Ther 7(4):829–840. doi:10.1158/1535-7163.MCT-07-0337
Calvo-Aller E, Baselga J, Glatt S, Cleverly A, Lahn M, Arteaga CL, Rothenberg ML, Carducci MA (2008) First human dose escalation study in patients with metastatic malignancies to determine safety and pharmacokinetics of LY2157299, a small molecule inhibitor of the transforming growth factor-beta receptor I kinase. J Clin Oncol 26(15S):14554, abstract
Cui Q, Lim SK, Zhao B, Hoffmann FM (2005) Selective inhibition of TGF-beta responsive genes by Smad-interacting peptide aptamers from FoxH1, Lef1 and CBP. Oncogene 24(24):3864–3874. doi:10.1038/sj.onc.1208556
Zhao BM, Hoffmann FM (2006) Inhibition of transforming growth factor-beta1-induced signaling and epithelial-to-mesenchymal transition by the Smad-binding peptide aptamer Trx-SARA. Mol Biol Cell 17(9):3819–3831. doi:10.1091/mbc.E05-10-0990
Chen D, Zhao M, Mundy GR (2004) Bone morphogenetic proteins. Growth Factors 22(4):233–241. doi:10.1080/08977190412331279890
ten Dijke P (2006) Bone morphogenetic protein signal transduction in bone. Curr Med Res Opin 22(Suppl 1):S7–S11. doi:10.1185/030079906X80576
Dudley AT, Lyons KM, Robertson EJ (1995) A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Gene Dev 9(22):2795–2807
Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G (1995) BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Gene Dev 9(22):2808–2820
Simic P, Vukicevic S (2005) Bone morphogenetic proteins in development and homeostasis of kidney. Cytokine Growth Factor Rev 16(3):299–308. doi:10.1016/j.cytogfr.2005.02.010
Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, Que I, Schwaninger R, Rentsch C, Ten Dijke P, Cleton-Jansen AM, Driouch K, Lidereau R, Bachelier R, Vukicevic S, Clezardin P, Papapoulos SE, Cecchini MG, Lowik CW, van der Pluijm G (2007) Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Canc Res 67(18):8742–8751. doi:10.1158/0008-5472.CAN-06-2490
Buijs JT, Petersen M, van der Horst G, van der Pluijm G (2010) Bone morphogenetic proteins and its receptors; therapeutic targets in cancer progression and bone metastasis? Curr Pharm Des 16(11):1291–1300
Buijs JT, Rentsch CA, van der Horst G, van Overveld PG, Wetterwald A, Schwaninger R, Henriquez NV, Ten Dijke P, Borovecki F, Markwalder R, Thalmann GN, Papapoulos SE, Pelger RC, Vukicevic S, Cecchini MG, Lowik CW, van der Pluijm G (2007) BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am J Pathol 171(3):1047–1057. doi:10.2353/ajpath.2007.070168
Gautschi OP, Frey SP, Zellweger R (2007) Bone morphogenetic proteins in clinical applications. ANZ J Surg 77(8):626–631. doi:10.1111/j.1445-2197.2007.04175.x
Institute AAMCTCNC (2009) Topical halofuginone hydrobromide in treating patients with HIV-related Kaposi’s sarcoma. National Cancer Institute (NCI). http://clinicaltrials.gov/ct2/show/NCT00064142, 2010 April 25
Juarez P, Chirgwin J, Mohammad KS, Ryan C, Walton H, Niewolna M, Mauviel A, Guise TA (2009) Halofuginone decreases osteolytic bone metastases. #MO0109. J Bone Miner Res 24:S402. doi:10.1002/jbmr.5650241305
Gadir N, Jackson DN, Lee E, Foster DA (2008) Defective TGF-beta signaling sensitizes human cancer cells to rapamycin. Oncogene 27(8):1055–1062. doi:10.1038/sj.onc.1210721
Filyak Y, Filyak O, Stoika R (2007) Transforming growth factor beta-1 enhances cytotoxic effect of doxorubicin in human lung adenocarcinoma cells of A549 line. Cell Biol Int 31(8):851–855. doi:10.1016/j.cellbi.2007.02.008
Taniguchi Y, Kawano K, Minowa T, Sugino T, Shimojo Y, Maitani Y (2010) Enhanced antitumor efficacy of folate-linked liposomal doxorubicin with TGF-beta type I receptor inhibitor. Canc Sci 101(10):7. doi:10.1111/j.1349-7006.2010.01646.x
Mohammad KS, Stebbins EG, Kingsley L, Fournier PGJ, Niewolna M, McKenna CR, Peng X, Higgins L, Wong D, Guise TA (2008) Combined transforming growth factor b receptor I kinase inhibitor and biphosphonates are additve to reduce breast cancer bone metastases. J Bone Miner Res 23:F275, Abstract
Won J, Kim H, Park EJ, Hong Y, Kim SJ, Yun Y (1999) Tumorigenicity of mouse thymoma is suppressed by soluble type II transforming growth factor beta receptor therapy. Canc Res 59(6):1273–1277
Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L (1989) Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol 142(5):1536–1541
Ganapathy V, Ge R, Grazioli A, Xie W, Banach-Petrosky W, Kang Y, Lonning S, McPherson J, Yingling JM, Biswas S, Mundy GR, Reiss M (2010) Targeting the transforming growth factor-beta pathway inhibits human basal-like breast cancer metastasis. Mol Canc 9:122. doi:10.1186/1476-4598-9-122
Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL (2007) Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Investig 117(5):1305–1313. doi:10.1172/JCI30740
Antisense Pharma (2008) Efficacy and safety of AP 12009 in patients with recurrent or refractory anaplastic astrocytoma (SAPPHIRE). National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00761280. Accessed 2011 Aug 23
Anitisense Pharma (2009) Safety and tolerability of AP 12009, administered I.V. in patients with advanced tumors known to overproduce TGF-beta-2. National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00844064. Accessed 2011 Aug 23
Lee GT, Hong JH, Mueller TJ, Watson JA, Kwak C, Sheen YY, Kim DK, Kim SJ, Kim IY (2008) Effect of IN-1130, a small molecule inhibitor of transforming growth factor-beta type I receptor/activin receptor-like kinase-5, on prostate cancer cells. J Urol 180(6):2660–2667. doi:10.1016/j.juro.2008.08.008
Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, Troconiz IF (2008) Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Canc 44(1):142–150. doi:10.1016/j.ejca.2007.10.008
Peng SB, Yan L, Xia X, Watkins SA, Brooks HB, Beight D, Herron DK, Jones ML, Lampe JW, McMillen WT, Mort N, Sawyer JS, Yingling JM (2005) Kinetic characterization of novel pyrazole TGF-beta receptor I kinase inhibitors and their blockade of the epithelial-mesenchymal transition. Biochemistry 44(7):2293–2304. doi:10.1021/bi048851x
Sawyer JS, Beight DW, Britt KS, Anderson BD, Campbell RM, Goodson T Jr, Herron DK, Li HY, McMillen WT, Mort N, Parsons S, Smith EC, Wagner JR, Yan L, Zhang F, Yingling JM (2004) Synthesis and activity of new aryl- and heteroaryl-substituted 5,6-dihydro-4H-pyrrolo[1,2-b]pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. Bioorg Med Chem Lett 14(13):3581–3584. doi:10.1016/j.bmcl.2004.04.007
Halder SK, Beauchamp RD, Datta PK (2005) A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia 7(5):509–521
Wiercinska E, Naber HP, Pardali E, van der Pluijm G, van Dam H, Ten Dijke P (2010) The TGF-beta/Smad pathway induces breast cancer cell invasion through the up-regulation of matrix metalloproteinase 2 and 9 in a spheroid invasion model system. Breast Canc Res Treat. doi:10.1007/s10549-010-1147-x
Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, Protter AA, Gauldie J (2005) Progressive transforming growth factor beta1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med 171(8):889–898. doi:10.1164/rccm.200405-612OC
Rausch MP, Hahn T, Ramanathapuram L, Bradley-Dunlop D, Mahadevan D, Mercado-Pimentel ME, Runyan RB, Besselsen DG, Zhang X, Cheung HK, Lee WC, Ling LE, Akporiaye ET (2009) An orally active small molecule TGF-beta receptor I antagonist inhibits the growth of metastatic murine breast cancer. Anticancer Res 29(6):2099–2109
Suzuki E, Kim S, Cheung HK, Corbley MJ, Zhang X, Sun L, Shan F, Singh J, Lee WC, Albelda SM, Ling LE (2007) A novel small-molecule inhibitor of transforming growth factor beta type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Canc Res 67(5):2351–2359. doi:10.1158/0008-5472.CAN-06-2389
Ishida W, Mori Y, Lakos G, Sun L, Shan F, Bowes S, Josiah S, Lee WC, Singh J, Ling LE, Varga J (2006) Intracellular TGF-beta receptor blockade abrogates Smad-dependent fibroblast activation in vitro and in vivo. J Invest Dermatol 126(8):1733–1744. doi:10.1038/sj.jid.5700303
Tran TT, Uhl M, Ma JY, Janssen L, Sriram V, Aulwurm S, Kerr I, Lam A, Webb HK, Kapoun AM, Kizer DE, McEnroe G, Hart B, Axon J, Murphy A, Chakravarty S, Dugar S, Protter AA, Higgins LS, Wick W et al (2007) Inhibiting TGF-beta signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol 9(3):259–270. doi:10.1215/15228517-2007-010
Wojtowicz-Praga S, Verma UN, Wakefield L, Esteban JM, Hartmann D, Mazumder A (1996) Modulation of B16 melanoma growth and metastasis by anti-transforming growth factor beta antibody and interleukin-2. J Immunother Emphasis Tumor Immunol 19(3):169–175
Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, Tong A, Kumar P, Pappen B, Hamilton C, DeVol E, Maples PB, Liu L, Chamberlin T, Shawler DL, Fakhrai H (2006) Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol 24(29):4721–4730. doi:10.1200/JCO.2005.05.5335
Nemunaitis J, Nemunaitis M, Senzer N, Snitz P, Bedell C, Kumar P, Pappen B, Maples PB, Shawler D, Fakhrai H (2009) Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Canc Gene Ther 16(8):620–624. doi:10.1038/cgt.2009.15
Phase I Trial of TGFB2-Antisense-GMCSF Gene Modified Autologous Tumor Cell (TAG) Vaccine for Advanced Cancer (Auto TAG) (2008) National Library of Medicine (US). http://clinicaltrials.gov/show/NCT00684294. Accessed Mar 11, 2011
Gorelik L, Flavell RA (2000) Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12(2):171–181
Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S (1993) Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 90(2):770–774
Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D et al (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359(6397):693–699. doi:10.1038/359693a0
Yaswen L, Kulkarni AB, Fredrickson T, Mittleman B, Schiffman R, Payne S, Longenecker G, Mozes E, Karlsson S (1996) Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse. Blood 87(4):1439–1445
Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL (2004) TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303(5659):848–851. doi:10.1126/science.1090922
Cheng N, Chytil A, Shyr Y, Joly A, Moses HL (2008) Transforming growth factor-beta signaling-deficient fibroblasts enhance hepatocyte growth factor signaling in mammary carcinoma cells to promote scattering and invasion. Mol Canc Res 6(10):1521–1533. doi:10.1158/1541-7786.MCR-07-2203
Prud’homme GJ (2007) Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Investig 87(11):1077–1091. doi:10.1038/labinvest.3700669
Klopcic B, Maass T, Meyer E, Lehr HA, Metzger D, Chambon P, Mann A, Blessing M (2007) TGF-beta superfamily signaling is essential for tooth and hair morphogenesis and differentiation. Eur J Cell Biol 86(11–12):781–799. doi:10.1016/j.ejcb.2007.03.005
Ruzek MC, Hawes M, Pratt B, McPherson J, Ledbetter S, Richards SM, Garman RD (2003) Minimal effects on immune parameters following chronic anti-TGF-beta monoclonal antibody administration to normal mice. Immunopharmacol Immunotoxicol 25(2):235–257
Siriwardena D, Khaw PT, King AJ, Donaldson ML, Overton BM, Migdal C, Cordeiro MF (2002) Human antitransforming growth factor beta(2) monoclonal antibody–a new modulator of wound healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology 109(3):427–431
Liu Z, Kobayashi K, van Dinther M, van Heiningen SH, Valdimarsdottir G, van Laar T, Scharpfenecker M, Lowik CW, Goumans MJ, Ten Dijke P, Pardali E (2009) VEGF and inhibitors of TGFbeta type-I receptor kinase synergistically promote blood-vessel formation by inducing alpha5-integrin expression. J Cell Sci 122(Pt 18):3294–3302. doi:10.1242/jcs.048942
Watabe T, Nishihara A, Mishima K, Yamashita J, Shimizu K, Miyazawa K, Nishikawa S, Miyazono K (2003) TGF-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J Cell Biol 163(6):1303–1311. doi:10.1083/jcb.200305147
Schlingensiepen KH, Schlingensiepen R, Steinbrecher A, Hau P, Bogdahn U, Fischer-Blass B, Jachimczak P (2006) Targeted tumor therapy with the TGF-beta2 antisense compound AP 12009. Cytokine Growth Factor Rev 17(1–2):129–139. doi:10.1016/j.cytogfr.2005.09.002
Bonafoux D, Lee WC (2009) Strategies for TGF-beta modulation: a review of recent patents. Expert Opin Ther Pat 19(12):1759–1769. doi:10.1517/13543770903397400
Hengst V, Oussoren C, Kissel T, Storm G (2007) Bone targeting potential of bisphosphonate-targeted liposomes. Preparation, characterization and hydroxyapatite binding in vitro. Int J Pharm 331(2):224–227. doi:10.1016/j.ijpharm.2006.11.024
Petersen M, Pardali E, van der Horst G, Cheung H, van den Hoogen C, van der Pluijm G, Ten Dijke P (2010) Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene 29(9):1351–1361. doi:10.1038/onc.2009.426
Dalal BI, Keown PA, Greenberg AH (1993) Immunocytochemical localization of secreted transforming growth factor-beta 1 to the advancing edges of primary tumors and to lymph node metastases of human mammary carcinoma. Am J Pathol 143(2):381–389
Shinto O, Yashiro M, Toyokawa T, Nishii T, Kaizaki R, Matsuzaki T, Noda S, Kubo N, Tanaka H, Doi Y, Ohira M, Muguruma K, Sawada T, Hirakawa K (2010) Phosphorylated smad2 in advanced stage gastric carcinoma. BMC Canc 10:652. doi:10.1186/1471-2407-10-652
Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J (2008) TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133(1):66–77. doi:10.1016/j.cell.2008.01.046
Acknowledgements
This work was supported by NIH grants R01CA69158, R01DK067333, R01DK065837 and U01CA143057; the Mary Kay Ash Foundation, the V-Foundation, the Jerry W. and Peggy S. Throgmartin Endowment of Indiana University and the Indiana Economic Development Fund (to T.A.G.), Department of Defense - Prostate Cancer Research Program 2010 – Prostate Cancer Training Award (to J.B. and T.A.G.) as well as a grant from the Susan Komen Foundation (T.A.G.).
Potential Conflict of Interests
T.A.G.: Consultant for AMGEN, ROCHE and Novartis. Stock ownership of AMGEN.
J.T.B and K.R.S.: no potential conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buijs, J.T., Stayrook, K.R. & Guise, T.A. TGF-β in the Bone Microenvironment: Role in Breast Cancer Metastases. Cancer Microenvironment 4, 261–281 (2011). https://doi.org/10.1007/s12307-011-0075-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-011-0075-6