Abstract
Background
Dupuytren’s disease (DD) is a fibroproliferative pathology that affects the palmar aponeurosis causing the development of nodules and collagen cords and the progressive flexion of the fingers. The standard procedure is surgical fasciectomy, followed by high recurrence rates. Collagenase Clostridium histolyticum (CCH) injection represents an innovative noninvasive approach to the treatment of DD. This prospective study was designed to examine the efficacy and safety of CCH injection performed in the outpatient, using local anesthesia.
Materials and methods
Forty patients [32 metacarpophalangeal (MP), 8 proximal interphalangeal (PIP)] with Dupuytren’s contracture of at least 20° for MP joint and any degree for PIP joint were included. The mean age was 66. All joints were treated with a single vial of collagenase injection and manual breaking of the cord 24 h after. All adverse effects (AEs) were monitored. Patients were checked 7, 30, 90, and 180 days after the injection. Primary endpoint was a reduction in digit contracture within 0°–5° of normal extension. Secondary endpoints were the improvement of range of motion, the evaluation of AEs incidence, and cost-effectiveness of collagenase treatment.
Results
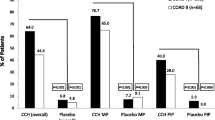
About 67.5 % of patients obtained a clinical success. At 6 months, a further 7.5 % attained the same result. The mean contracture of treated joints was 5.3º for MP and 6.8° for PIP joints. Twenty-three patients had one or more mild-to-moderate side effects.
Conclusions
The use of collagenase appears to be an effective and safe method for the treatment of Dupuytren’s contracture. Therapeutic success was achieved in a significant percentage of patients. The incidence of side effects was higher, but they were local reactions of short duration. The use of a single collagenase vial in patients treated in day surgery appears more cost-effective than surgery.



Similar content being viewed by others
Abbreviations
- CCH:
-
Collagenase Clostridium histolyticum
- MCP:
-
Metacarpophalangeal joint
- PIP:
-
Proximal interphalangeal joint
- PCRT:
-
Placebo-controlled randomized trial
- AE:
-
Adverse effects
References
Canale ST (2003) Campbell’s Operative Orthopaedics, vol 4, 10th edn.
Rayan GM (2007) Dupuytren’s disease: anatomy, pathology, presentation, and treatment. J Bone Joint Surg Am 89(1):189–198
Shaw RB Jr, Chong AK, Zhang A, Hentz VR, Chang J (2007) Dupuytren’s disease: history, diagnosis, and treatment. Plast Reconstr Surg 120(3):44–54
Smith AC (1991) Diagnosis and indications for surgical treatment. Hand Clin 7:635–642
Mafi R, Hindocha S, Khan W (2012) Recent surgical and medical advances in the treatment of Dupuytren’s disease—a systematic review of the literature. Open Orthop J 6(Suppl 1:M9):77–82
Crean SM, Gerber RA, Le Graverand MP, Boyd DM, Cappelleri JC (2011) The efficacy and safety of fasciectomy and fasciotomy for Dupuytren’s contracture in European patients: a structured review of published studies. J Hand Surg Eur Vol 36:396–407
Witthaut J, Jones G, Skrepnik N, Kushner H, Houston A, Lindau TR (2013) Efficacy and safety of collagenase Clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg 38(1):2-11
Chen NC, Srinivasan RC, Shauver MJ, Chung KC (2011) A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand 6(3):250–255
Werker PM, Pess GM, van Rijssen AL, Denkler K (2012) Correction of contracture and relapse rates of Dupuytren contracture following invasive treatment: the importance of clear definitions. J Hand Surg Am 37(10):2095–2105
Tripoli M, Cordova A, Moschella F (2010) The ‘‘Jacobsen Flap’’ technique: a safe, simple surgical procedure to treat Dupuytren disease of the little finger in advanced stage. Tech Hand Up Extrem Surg 14:173–177
Jacobsen K, Holst-Nielsen F (1977) A modified Mc Cash operation for Dupuytren’s contracture. Scand J Plast Reconstr Surg 11(3):231–233
Donaldson J, Goddar N (2012) The re-emergence of Percutaneous Fasciotomy in the Management of Dupuytren’s disease. Open Orthop J 6(Suppl 1: M10):83–87
Thomas A, Bayat A (2010) The emerging role of Clostridium histolyticum collagenase in the treatment of Dupuytren disease. Therap Clin Risk Manag 6:557–572
Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J, CORD I Study Group (2009) Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 361(10):968–979
Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N (2010) Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am 35(12):2027–2038
Peimer CA, Blazar P, Coleman S, Thomas F, Kaplan D, Smith T, Tursi JP, Cohen B, Kaufman GJ, Lindau T (2013) Dupuytren contracture relapse following treatment with collagenase Clostridium histolyticum (CORDLESS Study): 3-year data. J Hand Surg 38(1):12–22
Foissac R, Camuzard O, Dumas P, Dumontier C, Chignon-Sicard B (2013) Treatment of Dupuytren’s contracture by collagenase injection. Chir Main 32(4):199–205
Witthaut J, Jones G, Skrepnik N, Kushner H, Houston A, Lindau TR (2013) Efficacy and safety of collagenase Clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am 38(1):2–11
Bainbridge C, Gerber RA, Szczypa PP, Smith T, Kushner H, Cohen B, Hellio Le Graverand-Gastineau MP (2012) Efficacy of collagenase in patients who did and did not have previous hand surgery for Dupuytren’s contracture. J Plast Surg Hand Surg 46(3–4):177–183
Badalamente MA, Hurst LC (2007) Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am 32(6):767–774
Watt AJ, Curtin CM, Hentz VR (2010) Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J Hand Surg Am 35(4):534–539
Badalamente MA, Hurst LC (1996) Enzyme injection as a nonoperative treatment for Dupuytren’s disease. Drug Deliv 3:35–40
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alberton, F., Corain, M., Garofano, A. et al. Efficacy and safety of collagenase Clostridium histolyticum injection for Dupuytren contracture: report of 40 cases. Musculoskelet Surg 98, 225–232 (2014). https://doi.org/10.1007/s12306-013-0304-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-013-0304-x