Abstract
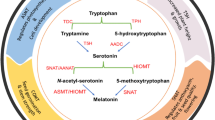
Melatonin (MEL) is the potential biostimulator molecule, governing multiple range of growth and developmental processes in plants, particularly under different environmental constrains. Mainly, its role is considered as an antioxidant molecule that copes with oxidative stress through scavenging of reactive oxygen species and modulation of stress related genes. It also enhances the antioxidant enzyme activities and thus helps in regulating the redox hemostasis in plants. Apart from its broad range of antioxidant functions, it is involved in the regulation of various physiological processes such as germination, lateral root growth and senescence in plants. Moreover this multifunctional molecule takes much interest due to its recent identification and characterization of receptorCandidate G-protein-Coupled Receptor 2/Phytomelatonin receptor(CAND2/PMTR1) in Arabidopsis thaliana. In this compiled work, different aspects of melatonin in plants such as melatonin biosynthesis and detection in plants, signaling pathway, modulation of stress related genes and physiological role of melatonin under different environmental stresses have been dissected in detail.



Similar content being viewed by others
References
Afreen F, Zobayed SM, Kozai T (2006) Melatonin in glycyrrhizauralensis: response of plant roots to spectral quality of light and UV-B radiation. J Pineal Res 41:108–115
Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA (2003) The chemistry of melatonin’s interaction with reactive species. J Pineal Res 34:1–10
Arnao MB (2014) Phytomelatonin: discovery, content, and role in plants. Adv Bot. https://doi.org/10.1155/2014/815769
Arnao MB, Hernández-Ruiz J (2007) Melatonin promotes adventitious-and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J Pineal Res 42:147–152
Arnao MB, Hernández-Ruiz J (2009a) Assessment of different sample processing procedures applied to the determination of melatonin in plants. Phytochem Anal 20:14–18
Arnao MB, Hernández-Ruiz J (2009b) Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J Pineal Res 46:58–63. https://doi.org/10.1111/j.1600-079x.2008.00625.x
Arnao MB, Hernández-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulatorduring stress? Trends Plant Sci 19:789–797
Arnao MB, Hernández-Ruiz J (2017a) Growth activity, rooting capacity, and tropism: three auxinic precepts fulfilled by melatonin. Acta Physiol Plant 39:127
Arnao MB, Hernández-Ruiz J (2017b) Melatonin and its relationship to plant hormones. Ann Bot 121:195–207
Arnao MB, Hernández-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci 24:38–48. https://doi.org/10.1016/j.tplants.2018.10.010
Bai Y, Guo J, Reiter RJ, Wei Y, Shi H (2020) Melatonin synthesis enzymes interact with ascorbate peroxidase to protect against oxidative stress in cassava. J Exp Bot. https://doi.org/10.1093/jxb/eraa267
Bajwa VS, Shukla MR, Sherif SM, Murch SJ, Saxena PK (2014) Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J Pineal Res 56:238–245
Bidabadi SS, VanderWeide J, Sabbatini P (2020) Exogenous melatonin improves glutathione content, redox state and increases essential oil production in two salvia species under drought stress. Sci Rep 10:1–12
Boccalandro HE, González CV, Wunderlin DA, Silva MF (2011) Melatonin levels, determined by LC-ESI-MS/MS, fluctuate during the day/night cycle in Vitis vinifera cv Malbec: evidence of its antioxidant role in fruits. J Pineal Res 51:226–232
Byeon Y, Back K (2014) Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotoninmethyltransferase activities. J Pineal Res 56:189–195
Campos CN, Ávila RG, deSouza KRD, Azevedo LM, Alves JD (2019) Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric Water Manag 211:37–47. https://doi.org/10.1016/j.agwat.2018.09.025
Cao YY, Qi CD, Li S, Wang Z, Wang X, Wang J, Ren S, Li X, Zhang N, Guo YD (2019) Melatonin alleviates copper toxicity via improving copper sequestration and ROS scavenging in cucumber. Plant Cell Physiol 60:562–574
Carrillo-Vico A, Lardone PJ, Álvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM (2013) Melatonin: buffering the immune system. Int J MolSci 14:8638–8683
Castanares JL, Bouzo CA (2019) Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic Plant J5:79–87
Chen Q, Qi WB, Reiter RJ, Wei W, Wang BM (2009) Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J Plant Phyiol 166:324–328
Chen YE, Mao JJ, Sun LQ, Huang B, Ding CB, Gu Y, Liao JQ, Hu C, Zhang ZW, Yuan S, Yuan M (2018) Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol Plant 164:349–363
Choi GH, Lee HY, Back K (2017) Chloroplast overexpression of rice caffeic acid O-methyltransferase increases melatonin production in chloroplasts via the 5-methoxytryptamine pathway in transgenic rice plants. J Pineal Res 63:12412
Cui G, Zhao X, Liu S, Sun F, Zhang C, Xi Y (2017) Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol Biochem 118:138–149
Dawood MG (2018) Physiological effect of melatonin, IAA and their precursor on quality and quantity of chickpea plants grown under sandy soil conditions. Agric Eng Int CIGR J 19:35–44
Debnath B, Hussain M, Irshad M, Mitra S, Li M, Liu S, Qiu D (2018) Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molecules 23:388. https://doi.org/10.3390/molecules23020388
Debnath B, Islam W, Li M, Sun Y, Lu X, Mitra S, Hussain M, Liu S, Qiu D (2019) Melatonin mediates enhancement of stress tolerance in plants. Int J Mol Sci 20:1040
De-Luca V, Marineau C, Brisson N (1989) Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylases. ProcNat lAcadSci 86:2582–2586
Di-Fiore S, Li Q, Leech MJ, Schuster F, Emans N, Fischer R, Schillberg S (2002) Targeting tryptophan decarboxylase to selected subcellular compartments of tobacco plants affects enzyme stability and in vivo function and leads to a lesion-mimic phenotype. Plant Physiol 129:1160–1169
Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W (1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res 18:28–31
Falcón J, Besseau L, Fuentès M, Sauzet S, Magnanou E, Boeuf G (2009) Structural and functional evolution of the pineal melatonin system in vertebrates. Annals NY AcadSci 1163:101–111
Fan J, Xie Y, Zhang Z, Chen L (2018) Melatonin: a Multifunctional Factor in Plants. Int J MolSci 19:1528
Farouk S, Al-Amri SM (2019) Exogenous melatonin-mediated modulation of arsenic tolerance with improved accretion of secondary metabolite production, activating antioxidant capacity and improved chloroplast ultrastructure in rose mary herb. Ecotoxicol Environ Saf 180:333–347
Filippou P, Tanou G, Molassiotis A, Fotopoulos V (2013) Plant acclimation to environmental stress using priming agents. In: Gill SS, Tuteja N (eds) Plant acclimation to environmental stress. Springer, New York, pp 1–27
Fujiwara T, Maisonneuve S, Isshiki M, Mizutani M, Chen L, Wong HL, Kawasaki T, Shimamoto K (2010) Sekiguchi lesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice. J Biol Chem 285:11308
Galano A, Tan DX, Reiter R (2018) Melatonin: a versatile protector against oxidative DNA damage. Molecules 23:530
Gao W, Zhang Y, Feng Z, Bai Q, He J, Wang Y (2018) Effects of melatonin on antioxidant capacity in naked oat seedlings under drought stress. Molecules 23:1580
Gomez FJV, Hernández IG, Martinez LD, Silva MF, Cerutti S (2013) Analytical tools for elucidating the biological role of melatonin in plants by LC-MS/MS. Electrophoresis 34:1749–1756
Gomez FJ, Hernández IG, Cerutti S, Silva MF (2015) Solid phase extraction/cyclodextrin-modified micellarelectrokinetic chromatography for the analysis of melatonin and related indole compounds in plants. Microchem J 123:22–27
Gul M, Khan FA, Wani SA, Bhat SA, Mir SA, Malik AA, Kumar A, Narayan S, Stephen K, Lone SA (2018) Foliar application of melatonin modulates the growth and photosynthetic pigments in broccoli cv. Palam Samridhi. Skuast J Res 20:193–198
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford University Press, USA
Han QH, Huang B, Ding CB, Zhang ZW, Chen YE, Hu C, Zhou LJ, Huang Y, Liao JQ, Yuan S, Yuan M (2017) Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front Plant Sci 8:785. https://doi.org/10.3389/fpls.2017.00785
Hardeland R (1996) Ubiquitous melatonin-presence and effects in unicells, plants and animals. Trends Comp Biochem Physiol 2:25–45
Hardeland R (2012) Melatonin in aging and disease—multiple consequences of reduced secretion, options and limits of treatment. Aging Dis 3:194
Hardeland R (2013) Melatonin and the theories of aging: a critical appraisal of melatonin’s role in antiaging mechanisms. J Pineal Res 55:325–356
Hardeland R (2016) Melatonin in plants–diversity of levels and multiplicity of functions. Front Plant Sci 19:198
Hardeland R, Pandi-Perumal SR, Poeggeler B (2007) Melatonin in plants—focus on a vertebrate night hormone with cytoprotective properties. Funct Plant Sci Biotechnol 1:3245
Hasan M, Ahammed GJ, Yin L, Shi K, Xia X, Zhou Y, Yu J, Zhou J (2015a) Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front in Plant Sci 11:601
Hasan MK, Ahammed GJ, Yin L, Shi K, Xia X, Zhou Y et al (2015b) Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front Plant Sci 6:601
Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Bio Int 35:627–634
Hernández-Ruiz J, Cano A, Arnao MB (2005) Melatonin acts as a growth-stimulating compound in some monocot species. J Pineal Res 39:137–142
Huang X, Mazza G (2011a) Application of LC and LC-MS to the analysis of melatonin and serotonin in edible plants. Crit Rev Food Sci Nutr 51:269–284
Huang X, Mazza G (2011b) Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J Chromatogr 1218:3890–3899
Huang YH, Liu SJ, Yuan S, Guan C, Tian DY, Cui X, Zhang YW, Yang FY (2017) Overexpression of ovine AANAT and HIOMT genes in switchgrass leads to improved growth performance and salt-tolerance. Sci Rep 7:1–13
Kabiri R, Hatami A, Oloumi H, Naghizadeh M, Nasibi F, Tahmasebi Z (2018) Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic 30:155–167
Kang S, Kang K, Lee K, Back K (2007) Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 227:263–272
Kang K, Lee K, Park S, Kim YS, Back K (2010) Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J Pineal Res 49:176–182
Ke Q, Ye J, Wang B, Ren J, Yin L, Deng X, Wang S (2018) Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front Plant Sci 9:914. https://doi.org/10.3389/fpls.2018.00914
Kolář J, Macháčková I (2005) Melatonin in higher plants: occurrence and possible functions. J Pineal Res 39:333–341
Kołodziejczyk I, Dzitko K, Szewczyk R, Posmyk MM (2016) Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. J Plant Physiol 193:47–56
Lazar D, Murch SJ, Beilby MJ, Al Khazaaly S (2013) Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal Behav 8:23279. https://doi.org/10.4161/psb.23279
Lee K, Back K (2017) Overexpression of rice serotonin N-acetyltransferase 1 in transgenic rice plants confers resistance to cadmium and senescence and increases grain yield. J Pineal Res 62:12392. https://doi.org/10.1111/jpi12392
Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W (1958) Isolation of melatonin, the pineal gland factor that lightens melanocyte S1. J Am Chem Soc 80:2587
Lerner AB, Case JD, Mori W, Wright MR (1959) Melatonin in peripheral nerve. Nature 183:1821
Li C, Wang P, Wei Z, Liang D, Liu C, Yin L, Jia D, Fu M, Ma F (2012) The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res 53:298–306
Li D, Zhang D, Wang H, Li Y, Li R (2017a) Physiological response of plants to polyethylene glycol (PEG-6000) by exogenous melatonin application in wheat. Zemdirbyste-Agric 104:219–228
Li H, Xu H, Zhang P, Gao M, Wang D, Zhao H (2017b) High temperature effects on D1 protein turnover in three wheat varieties with different heat susceptibility. Plant Growth Regul 81:1–9
Li J, Yang Y, Sun K, Chen Y, Chen X, Li X (2019) Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 24:1826. https://doi.org/10.3390/molecules24091826
Liang C, Zheng G, Li W, Wang Y, Hu B, Wang H, Wu H, Qian Y, Zhu XG, Tan DX, Chen SY (2015) Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J Pineal Res 59:91–101
Lim PO, Kim HJ, Gil-Nam H (2007) Leaf senescence. Ann Rev Plant Biol 58:115–136
Liu J, Yang J, Zhang H, Cong L, Zhai R, Yang C, Wang Z, Ma F, Xu L (2019) Melatonin inhibits ethylene synthesis via nitric oxide regulation to delay postharvest senescence in pears. J Agric Food Chem 67:2279–2288. https://doi.org/10.1021/acs.jafc.8b06580
Ly D, Kang K, Choi JY, Ishihara A, Back K, Lee SG (2008) HPLC analysis of serotonin, tryptamine, tyramine, and the hydroxycinnamic acid amides of serotonin and tyramine in food vegetables. J Med Food 11:385–389
Mahal HS, Sharma HS, Mukherjee T (1999) Antioxidant properties of melatonin: a pulse radiolysis study. Free RadicBiol Med 26:557–565
Majidinia M, Reiter RJ, Shakouri SK (2018) The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Res Rev 47:198–213
Maronde E, Stehle JH (2007) The mammalian pineal gland: known facts, unknown facets. Trends Endocrinol Metab 18:142–149
Martinez V, Nieves-Cordones M, Lopez-Delacalle M, Rodenas R, Mestre TC, Garcia-Sanchez F, Rubio F, Nortes PA, Mittler R, Rivero RM (2018) Tolerance to stress combination in tomato plants: new insights in the protective role of melatonin. Molecules 23:535. https://doi.org/10.3390/molecules23030535
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Murch SJ, Saxena PK (2006) A melatonin-rich germplasm line of St John’s wort (Hypericum perforatum L). J Pineal Res 41:284–287. https://doi.org/10.1111/j.1600-079x.2006.00367.x
Murch SJ, KrishnaRaj S, Saxena PK (2000) Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep 19:698–704
Ozgur R, Uzilday B, Turkan I, Sekmen AH (2017) The effects of melatonin on transcriptional profile of unfolded protein response genes under endoplasmic reticulum stress in Arabidopsis thaliana. Plant Mol Biol Rep 35:188–202
Padumanonda T, Johns J, Sangkasat A, Tiyaworanant S (2014) Determination of melatonin content in traditional Thai herbal remedies used as sleeping aids. J Pharm Sci 22:6
Pape C, Lüning K (2006) Quantification of melatonin in phototrophic organisms. J Pineal Res 41:157–165
Park S, Kang K, Lee K, Choi D, Kim YS, Back K (2009) Induction of serotonin biosynthesis is uncoupled from the coordinated induction of tryptophan biosynthesis in pepper fruits (Capsicum annuum) upon pathogen infection. Planta 230:1197
Park S, Lee DE, Jang H, Byeon Y, Kim YS, Back K (2013) Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J Pineal Res 54:258–263
Poeggeler B, Reiter RJ, Hardeland R, Tan DX, Barlow-Walden LR (1996) Melatonin and structurally-related, endogenous indoles act as potent electron donors and radical scavengers in vitro. Redox Rep 2:179–184
Posmyk MM, Janas KM (2009) Melatonin in plants. Acta Physiol Plant 31:1
Posmyk MM, Kuran H, Marciniak K, Janas KM (2008) Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J Pineal Res 45:24–31
Qiao Y, Ren J, Yin L, Liu Y, Deng X, Liu P, Wang S (2020) Exogenous melatonin alleviates PEG-induced short-term water deficiency in maize by increasing hydraulic conductance. BMC Plant Bio 20:1–14
Ragab AS, Van Fleet J, Jankowski B, Park JH, Bobzin SC (2006) Detection and quantitation of resveratrol in tomato fruit (Lycopersicon esculentum Mill.). J Agric Food Chem 54:7175–7179
Reiter RJ (1991) Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev 12:151–180
Reiter RJ (2000) Melatonin: mechanisms and actions as an antioxidant. Curr Topics Biophy 24:171–183
Reiter RJ, Poeggeler B, Dun-xian T, Chen LD, Manchester LC, Guerrero JM (1993) Antioxidant capacity of melatonin: a novel action not requiring a receptor. Neuroendocrinol Lett 15:103–116
Reiter RJ, Tan DX, Osuna C, Gitto E (2000) Actions of melatonin in the reduction of oxidative stress. J Biomed Sci 7:444–458
Roberts JE, HU DN, Martinez L, Chignell CF (2000) Photophysical studies on melatonin and its receptor agonists. J Pineal Res 29:94–99
Romero A, Ramos E, deLosRíos C, Egea J, DelPino J, Reiter RJ (2014) A review of metal-catalyzed molecular damage: protection by melatonin. J Pineal Res 56:343–370
Sadak MS (2016) Mitigation of salinity adverse effects on wheat by grain priming with melatonin. Int J Chem Tech Res 9:85–97
Shi H, Chan Z (2014) The cysteine2/histidine2-type transcription factor zinc finger of arabidopsis thaliana 6-activated C-repeat-binding factor pathway is essential for melatonin-mediated freezing stress resistance in Arabidopsis. J Pineal Res 57:185–191
Shi H, Reiter RJ, Tan DX, Chan Z (2015a) INDOLE-3-ACETIC ACID INDUCIBLE 17 positively modulates natural leaf senescence through melatonin-mediated pathway in Arabidopsis. J Pineal Res 58(1):26–33
Shi H, Tan DX, Reiter RJ, Ye T, Yang F, Chan Z (2015b) Melatonin induces class A1 heat-shock factors (HSFA 1 s) and their possible involvement of thermotolerance in Arabidopsis. J Pineal Res 58:335–342
Simopoulos AP, Tan DX, Manchester LC, Reiter RJ (2005) Purslane: a plant source of omega-3 fatty acids and melatonin. J Pineal Res 39:331–332
Sliwinski T, Rozej W, Morawiec-Bajda A, Morawiec Z, Reiter R, Blasiak J (2007) Protective action of melatonin against oxidative DNA damage—chemical inactivation versus base-excision repair. Mutat Res, Genet Toxicol Environ Mutagen 634:220–227
Srinivasan V, Pandi-Perumal SR, Maestroni GJM, Esquifino AI, Hardeland R, Cardinali DP (2005) Role of melatonin in neurodegenerative diseases. Neurotox Res 7:293–318
Stasica P, Ulanski P, Rosiak J (1998) Reactions of melatonin with radicals in deoxygenated aqueous solution. J Radioanal Nucl Chem 232:107–113
Stehle JH, Saade A, Rawashdeh O, Ackermann K, Jilg A, Sebestény T, Maronde E (2011) A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res 51:17–43
Szafrańska K, Reiter RJ, Posmyk MM (2016) Melatonin application to Pisumsativum L seeds positively influences the function of the photosynthetic apparatus in growing seedlings during paraquat-induced oxidative stress. Front Plant Sci 7:1663
Tan DX (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J 1:57–60
Tan DX, Manchester LC, Reiter RJ, Plummer BF, Limson J, Weintraub ST, Qi W (2000) Melatonin directly scavenges hydrogen peroxide: a potentially new metabolic pathway of melatonin biotransformation. Free Radical Bio Med 29:1177–1185
Tan DX, Manchester LC, Burkhardt S, Sainz RM, Mayo JC, Kohen R, Shohami E, Huo YS, Hardeland R, Reiter RJ (2001) N 1-acetyl-N 2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J 15:2294–2296
Tan DX, Reiter RJ, Manchester LC, Yan MT, El-SawiM Sainz RM, Mayo JC, Kohen R, Allegra MC, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2:181–197
Tan DX, Manchester LC, Helton P, Reiter RJ (2007) Phytoremediative capacity of plants enriched with melatonin. Plant Signa lBehav 2:514–516
Tan DX, Hardeland R, Manchester LC, Rosales-Corral S, Coto-Montes A, Boga JA, Reiter RJ (2012) Emergence of naturally occurring melatonin isomers and their proposed nomenclature. J Pineal Res 53:113–121
Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20:18886–18906
Tan DX, Hardeland R, Back K, Manchester LC, Alatorre-Jimenez MA, Reiter RJ (2016) On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. J Pineal Res 61:27–40
Thomas JC, Adams DG, Nessler CL, Brown JK, Bohnert HJ (1995) Tryptophan decarboxylase, tryptamine, and reproduction of the whitefly. Plant Physiol 109:717–720. https://doi.org/10.1104/pp.109.2.717
Tiryaki I, Keles H (2012) Reversal of the inhibitory effect of light and high temperature on germination of Phacelia tanacetifolia seeds by melatonin. J Pineal Res 52:332–339
Van-Tassel DL, O’neill SD (2001) Putative regulatory molecules in plants: evaluating melatonin. J Pineal Res 31:1–7
Wan J, Zhang P, Wang R, Sun L, Ju Q, Xu J (2018) Comparative physiological responses and transcriptome analysis reveal the roles of melatonin and serotonin in regulating growth and metabolism in Arabidopsis. BMC Plant Biol 18:362
Wang P, Yin L, Liang D, Li C, Ma F, Yue Z (2012) Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate–glutathione cycle. J Pineal Res 53:11–20
Wang P, Sun X, Chang C, Feng F, Liang D, Cheng L, Ma F (2013a) Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J Pineal Res 55:424–434. https://doi.org/10.1111/jpi.1209
Wang P, Sun X, Li C, Wei Z, Liang D, Ma F (2013b) Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pineal Res 54:292–302
Wang L, Zhao Y, Reiter RJ, He C, Liu G, Lei Q, Zuo B, Zheng XD, Li Q, Kong J (2014) Changes in melatonin levels in transgenic ‘Micro-Tom’tomato overexpressing ovine AANAT and ovine HIOMT genes. J Pineal Res 56:134–142
Wang LY, Liu JL, Wang WX, Sun Y (2016) Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 54:19–27
Weeda S, Zhang N, Zhao X, Ndip G, Guo Y, Buck GA, Fu C, Ren S (2014) Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 9:93462
Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, Zhang WK, Ma B, Lin Q, Zhang JS, Chen SY (2014) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 66:695–707. https://doi.org/10.1093/jxb/eru392
Wei J, Li DX, Zhang JR, Shan C, Rengel Z, Song ZB, Chen Q (2018) Phytomelatonin receptor PMTR 1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J Pineal Res 65:12500
Wen D, Gong B, Sun S, Liu S, Wang X, Wei M, Yang F, Li Y, Shi Q (2016) Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L by regulating auxin and nitric oxide signaling. Front Plant Sci 7:718
Wolf K, Kolář J, Witters E, van Dongen W, van Onckelen H, Macháčková I (2001) Daily profile of melatonin levels in Chenopodium rubrum L. depends on photoperiod. J Plant Physiol 158:1491–1493
Xia H, Ni Z, Pan D (2017) Effects of exogenous melatonin on antioxidant capacity in Actinidia seedlings under salt stress. In: IOP conference series: earth environ sci, vol 1. IOP Publishing, p 012024
Xu W, Cai SY, Zhang Y, Wang Y, Ahammed GJ, Xia XJ, Shi K, Zhou YH, Yu JQ, Reiter RJ, Zhou J (2016) Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J Pineal Res 61:457–469
Yang XL, Xu H, Li D, Gao X, Li TL, Wang R (2018) Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica. https://doi.org/10.1007/s11099-017-0748-6
Yang WJ, Du YT, Zhou YB, Chen J, Xu ZS, Ma YZ, Chen M, Min DH (2019) Overexpression of TaCOMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis. Int J MolSci 20:652. https://doi.org/10.3390/ijms20030652
Ye J, Wang S, Deng X, Yin L, Xiong B, Wang X (2016) Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol Plant 38:48
Ye T, Hao YH, Yu L, Shi H, Reiter RJ, Feng YQ (2017) A simple, rapid method for determination of melatonin in plant tissues by UPLC coupled with high resolution Orbitrap mass spectrometry. Front Plant Sci 8:64
Zeng Liu, Cai JS, Li JJ, Gy Lu, Li CS, Fu GP, Zhang XK, Liu QY, Zou XL, Cheng Y (2018) Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. J Integ Agri 17:328–335
Zettersten C, Co M, Wende S, Turner C, Nyholm L, Sjōberg PJ (2009) Identification and characterization of polyphenolic antioxidants using on-line liquid chromatography, electrochemistry, and electrospray ionization tandem mass spectrometry. Anal Chem 81:8968–8977
Zhang L, Jia J, Xu Y, Wang Y, Hao J, Li T (2012) Production of transgenic nicotianasylvestrisplants expressing melatonin synthetase genes and their effect on UV-B-induced DNA damage. In Vitro Cell Dev Bio Plant 48:275–282
Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Ren S, Guo YD (2013) Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J Pineal Res 54:15–23. https://doi.org/10.1111/j.1600-079x.2012.01015.x
Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S, Guo YD (2015) Roles of melatonin in abiotic stress resistance in plants. J Exp Bot 66:647–656. https://doi.org/10.1093/jxb/eru336
Zhang N, Sun Q, Li H, Li X, Cao Y, Zhang H, Li S, Zhang L, Qi Y, Ren S, Zhao B (2016) Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front Plant Sci 7:197. https://doi.org/10.3389/fpls.2016.00197
Zhang N, Zhang HJ, Sun QQ, Cao YY, Li X, Zhao B, Wu P, Guo YD (2017a) Proteomic analysis reveals a role of melatonin in promoting cucumber seed germination under high salinity by regulating energy production. Sci Rep 7:503
Zhang YP, Yang SJ, Chen YY (2017b) Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Bio Plant 61:571–578. https://doi.org/10.1007/s10535-017-0717-8
Zhao Y, Tan DX, Lei Q, Chen H, Wang L, Li QT, Gao Y, Kong J (2013) Melatonin and its potential biological functions in the fruits of sweet cherry. J Pineal Res 55:79–88
Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol 10:249
Zheng X, Tan DX, Allan AC, Zuo B, Zhao Y, Reiter RJ, Wang L, Wang Z, Guo Y, Zhou J, Shan D (2017) Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci Rep 7:41236
Zuo BX, Zheng XD, He PL, Wang L, Lei Q, Feng C et al (2014) Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J Pineal Res 57:408–417. https://doi.org/10.1111/jpi.12180
Acknowledgement
Tanveer Ahmad Khan acknowledges financial support from CSIR-UGC (Council of Scientific & Industrial Research, University Grants Commission) New Delhi, India as Junior Research fellowship under Ref. No. (553/CSIR-UGC NET) at Aligarh Muslim University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, T.A., Fariduddin, Q., Nazir, F. et al. Melatonin in business with abiotic stresses in plants. Physiol Mol Biol Plants 26, 1931–1944 (2020). https://doi.org/10.1007/s12298-020-00878-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-020-00878-z