Abstract
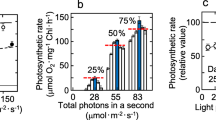
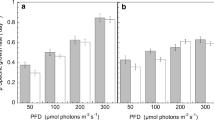
Effect of temperatures and illumination of temperate winter on photosynthesis and respiration was studied in the psychrophilic microalgae, Koliella antarctica (Trebouxiophyceae). Outdoor and indoor algal cultures were compared. Photosynthetic as well as respiration rates increased as light and temperature increased, until 35 °C, more in outdoor than in indoor cells, in agreement with the calculated Q10 values. K. antarctica showed important strategy mechanisms of adaption to the several temperature and light conditions. These significant photo-acclimation and thermo-acclimation abilities make it possible to cultivate Koliella for different uses, under less expensive outdoor conditions. Therefore, K. antarctica shows important strategy mechanisms of adaption to various temperature and light conditions; moreover, by varying the culture conditions, it is possible to modulate and optimize the growth and accordingly the biomass production. This is a very interesting point since it has been proved that this microalga is a promising potential source of functional ingredients, such as polyunsaturated fatty acids and carotenoids, suitable for industrial purposes.





Similar content being viewed by others
References
Andreoli C, Lokhrost GM, Mani AM, Scarabel L, Moro I, La Rocca N, Tognetto L (1998) Koliella antarctica sp. nov. (Klebsormidiales) a new marine green microalga from the Ross Sea (Antarctica). Arch Hydrobiol Algol Stud 90:1–8
Andreoli C, Moro I, La Rocca N, Dalla Valle L, Masiero L, Rascio N, Dalla Vecchia F (2000) Ecological, physiological, and biomolecular survey on microalgae from Ross sea (Antartica). Ital J Zool 67(suppl):147–156
Arrigo KR, Weiss AM, Smith WO (1998) Physical forcing of phytoplankton dynamics in the southwestern Ross Sea. J Geophys Res 103:1007–1021
Cid-Agüero P, Cárdenas PO, Moreno JD (2012) Growth response of Antarctic snow microalgae cultures belonging to the Chlamidomonadaceae family to the effects of temperature, irradiance and supporting media. An Inst Patagonia (Chile) 40(1):153–156
Coles JF, Jones RC (2000) Effect of temperature on photosynthesis-light response and growth of four phytoplankton species isolated from a tidal freshwater river. J Phycol 36:7–16
Colla LM, Oliveira Reinehr C, Reichert C, Vieira Costa JA (2007) Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regime. Bioresour Technol 98:1489–1493
Davison IR (1991) Environmental effects on algal photosynthesis: temperature. J Phycol 27:2–8
Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1(3):200–208
Ferrara M, Guerriero G, Cardi M, Esposito S (2012) Purification and biochemical characterisation of a glucose-6-phosphate dehydrogenase from the psychrophilic green alga Koliella antarctica. Extremophiles 17(1):53–62
Ferroni L, Baldisserotto C, Zennaro V, Soldani C, Fasulo MP, Pancaldi S (2006) Acclimation to darkness in the marine chlorophyte Koliella antarctica cultured under low salinity: hypothesis on its origin in the polar environment. Eur J Phycol 41(4):1–14
Fogliano V, Andreoli C, Martello A, Caiazzo M, Lobosco O, Formisano F, Carlino PA, Meca G, Graziani G, Di Martino Rigano V, Vona V, Carfagna S, Rigano C (2010) Functional ingredients produced by culture of Koliella antartica. Aquaculture 299:115–120
Geider RJ, Osborne BA (1992) Algal photosynthesis, the measurement of algal gas exchange. Chapman and Hall, London, p 256
Grobbelaar JU, Kurano N (2003) Use of photoacclimation in the design of a novel photobioreactor to achieve high yields in algal mass cultivation. J Appl Phycol 15:121–126
Inskeep WP, Bloom PR (1985) Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol 77:483–485
Kvìderovà J, Shukla SP, Pushparaj B, Elster J (2017) Perspectives of low-temperature biomass production of polar microalgae and biotechnology expansion into high latitudes. From Biodiversity to Biotechnology, Psychrophiles, pp 585–600
La Rocca N, Sciuto K, Meneghesso A, Moro I, Rascio N, Morosinotto T (2014) Photosynthesis in extreme environments: responses to different light regimes in the Antarctic alga Koliella antarctica. Physiol Plant 153(4):654–667
Lyon BR, Mock T (2014) Polar microalgae: new approaches towards understanding adaption to an extreme and changing environment. Biology (Basel) 3(1):56–80
Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20(20):5587–5594
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Quinn JC, Yates T, Douglas N, Weyer K, Butler J, Bradley TH, Lammers PJ (2012) Nannochloropsis production metrics in a scalable outdoor photobioreactor for commercial applications. Bioresour Technol 117:164–171
Rezanka T, Nedbalová L, Sigler K (2008) Unusual medium-chain polyunsaturated fatty acids from the snow alga Chloromonas brevispina. Microbiol Res 163(4):373–379
Richmond A, Boussiba S, Vonshak A, Kopel R (1993) A new tubular reactor for mass production of microalgae outdoors. J Appl Phycol 5:327–332
Rigano C, Di Martino Rigano V, Vona V, Esposito S, Di Martino C (1993) Effect of inhibitors on ammonium assimilation in Chlorella sorokiniana in light and darkness. Physiol Plant 89:602–606
Rivas C, Navarro N, Huovinen P, Gómez I (2016) Photosynthetic UV stress tolerance of the Antarctic snow alga Chlorella sp. modified by enhanced temperature? Revista Chilena de Historia Natural 89(7):1–9
Sato T, Usui S, Tsuchiya Y, Kondo Y (2006) Invention of outdoor closed type photobioreactor for microalgae. Energy Convers Manag 47:791–799
Thomas DN, Dieckmann GS (2002) Antarctic sea ice—a habitat for extremophiles. Science 295:641–644
Thomas H, Ougham H, Hortensteiner S (2001) Recent advances in the cell biology of chlorophyll catabolism. Adv Bot Res 35:1–52
Varshney P, Mikulic P, Vonshak A, Beardall J, Wangikar PP (2015) Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol 184:363–372
Vona V, Di Martino Rigano V, Lobosco O, Carfagna S, Esposito S, Rigano C (2004) Temperature responses of growth, photosynthesis, respiration and NADH: nitrate reductase in the cryophilic and mesophilic algae. New Phytol 163:325–331
Vonshak A, Torzillo G, Masojidek J, Boussiba S (2001) Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta). Plant Cell Environ 24:1113–1118
Webb WL, Newton M, Starr D (1974) Carbon dioxide exchange of Alnus rubra. Oecologia 17:281–291
Whitelam GC, Codd GA (1986) Damaging effects of light on microorganisms. In: Herbert RA, Codd GA (eds) Microbes in extreme environments. Academic Press, London, pp 129–169
Acknowledgements
The authors are very grateful to Professor Petronia Carillo for critical reading of the manuscript. This work was supported by a Grant from Regione Campania, Law 5/2002, year 2007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vona, V., Di Martino Rigano, V., Andreoli, C. et al. Comparative analysis of photosynthetic and respiratory parameters in the psychrophilic unicellular green alga Koliella antarctica, cultured in indoor and outdoor photo-bioreactors. Physiol Mol Biol Plants 24, 1139–1146 (2018). https://doi.org/10.1007/s12298-018-0595-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-018-0595-3