Abstract
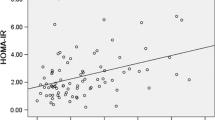
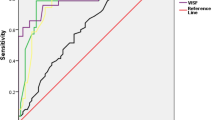
Gestational diabetes mellitus (GDM) is a common medical complication associated with pregnancy. The present study evaluates the changes in maternal adipocytokines (leptin, adiponectin, resistin, visfatin and tumor necrosis factor-alpha; TNF-α) in pregnancy complicated with GDM compared to normal pregnancy at 2nd and 3rd trimesters. The study included total number of 142 pregnant women classified into 4 groups: normal pregnancy (n = 33) and pregnancy with GDM (n = 24) both at 2nd trimester and normal pregnancy (n = 38) and GDM (n = 47) at 3rd trimester. Both GDM groups were significantly presented with elevated body mass index, fasting blood sugar and abnormal oral glucose tolerance test compared to their matched control. Results indicated reduction in maternal serum leptin and adiponectin in GDM compared to normal pregnancy at 3rd trimester. Elevated resistin and TNF-α were evident among pregnancy complicated with GDM at both tested trimesters. On the other hand, significant elevation in maternal visfatin was noted between GDM and matched control at 2nd trimester only. Significant increase in maternal leptin and visfatin and resistin was noted by advances in gestational period in healthy pregnancy. On the other hand, reduced adiponectin and elevated visfatin mean values were noticed in GDM at 3rd compared to 2nd trimester. It could be concluded that increased insulin resistance accompanies GDM is associated with suppressed leptin and adiponectin and increased resistin and TNF-α which might suggest their involvement in the development of GDM.
Similar content being viewed by others
References
Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988;67:341–7.
Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–16.
Homko CJ, Sivan E, Reece EA, Boden G. Fuel metabolism during pregnancy. Semin Reprod Endocrinol. 1999;17:119–25.
Catalano PM, Nizielski SE, Shao J, Preston L, Qiao L, Friedman JE. Downregulated IRS-1 and PPARgamma in obese women with gestational diabetes: relationship to FFA during pregnancy. Am J Physiol Endocrinol Metab. 2002;282:E522–33.
Friedman JE, Ishizuka T, Shao J, Huston L, Highman T, Catalano P. Impaired glucose transport and insulin receptor tyrosine phosphorylation in skeletal muscle from obese women with gestational diabetes. Diabetes. 1999;48:1807–14.
American Diabetes Association. Consensus Development Conference on Insulin Resistance. 5–6 November 1997. Diabet Care 1998;21:310–314.
Conway DL, Langer O. Effects of new criteria for type 2 diabetes on the rate of postpartum glucose intolerance in women with gestational diabetes. Am J Obst Gynecol. 1999;181:610–4.
Al-Rowaily MA, Abolfotouh MA. Predictors of gestational diabetes mellitus in a high-parity community in Saudi Arabia. East Mediterr Health J. 2010;16:636–41.
Perkins JM, Dunn JP, Jagasia SM. Perspectives in gestational diabetes mellitus: a review of screening, diagnosis and treatment. Clin Diabete. 2007;25:57–62.
Briana DD, Malamitsi-Puchner A. Adipocytokines in normal and complicated pregnancies. Reprod Sci. 2009;16:921–37.
Lappas M, Permezel M, Rice GE. Leptin and adiponectin stimulate the release of proinflammatory cytokines and prostaglandins from human placenta and maternal adipose tissue via nuclear factor-kB, peroxismal proliferators-activated receptor-γ and extracellularly regulated kinase. Endocrinology. 2005;146:3334–42.
Valsamakis G, Kumar S, Creatsas G, Mastorakos G. The effects of adipose tissue and adipocytokines in human pregnancy. Ann N Y Acad Sci. 2010;1205:76–81.
World Health Organization. WHO Consultation: Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus, Geneva, 1999; WHO/NCD/NCS/99.2.
Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:989–93.
Gao XL, Yang HX, Zhao Y. Variations of tumor necrosis factor-alpha, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chinese Med J. 2008;121:701–5.
Festa A, Shnawa N, Krugluger W, Hopmeier P, Schernthaner G, Haffner SM. Relative hypoleptinaemia in women with mild gestational diabetes mellitus. Diabet Med. 1999;16:656–62.
Skvarca A, Tomazic M, Krhin B, Blagus R, Janez A. Adipocytokines and insulin resistance across various degrees of glucose tolerance in pregnancy. J Int Med Res. 2012;40:583–9.
Challier J, Galtier M, Bintein T, Cortez A, Lepercq J, Hauguel-de Mouzon S. Placental leptin receptor isoforms in normal and pathological pregnancies. Placenta. 2003;24:92–9.
Henson MC, Castracane VD. Leptin: roles and regulation in primate pregnancy. Semin Reprod Med. 2002;20:113–22.
Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–33.
Highman TJ, Friedman JE, Huston LP, Wong WW, Catalano PM. Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy. Am J Obstet Gynecol. 1998;178:1010–15.
Mastorakos G, Valsamakis G, Papatheodorou DC, Barlas I, Margeli A, Boutsiadis A, et al. The role of adipocytokines in insulin resistance in normal pregnancy: visfatin concentrations in early pregnancy predict insulin sensitivity. Clin Chem. 2007;53:1477–83.
Christou H, Serdy S, Mantzoros CS. Leptin in relation to growth and developmental processes in the fetus. Semin Reprod Med. 2002;20:123–30.
Linnemann K, Malek A, Sager R, Blum WF, Schneider H, Fusch C. Leptin production and release in the dually in vitro perfused human placenta. J Clin Endocrinol Metab. 2000;85:4298–301.
Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol. 2006;194:1537–45.
Nuamah MA, Yura S, Sagawa N, Itoh H, Mise H, Korita D, et al. Significant increase in maternal plasma leptin concentration in induced delivery: a possible contribution of pro-inflammatory cytokines to placental leptin secretion. Endocr J. 2004;51:177–87.
Schulz LC, Townsend K, Kunz TH, Widmaier EP. Inhibition of trophoblast invasiveness in vitro by immunoneutralization of leptin in the bat, Myotis lucifugus (Chiroptera). Gen Comp Endocrinol. 2007;150:59–65.
Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, et al. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 2006;49:1292–302.
Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, Cozzi V, et al. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol. 2007;66:447–53.
Williams MA, Qiu C, Muy-Rivera M, Vadachkoria S, Song T, Luthy DA. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89:2306–11.
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6.
Pittas AG, Joseph NA, Greenberg AS. Hot topic: adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004;89:447–52.
Hulver MW, Saleh O, MacDonald KG, Pories WJ, Barakat HA. Ethnic differences in adiponectin levels. Metabolism. 2004;53:1–3.
Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPAR-γ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9.
Thyfault JP, Hedberg EM, Anchan RM, Thorne OP, Isler CM, Newton ER, et al. Gestational diabetes is associated with depressed adiponectin levels. J Soc Gynecol Investig. 2005;12:41–5.
Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–7.
Beltowski J. Adiponectin and resistin—new hormones of white adipose tissue. Med Sci Monitor. 2003;9:RA55–61.
Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–85.
Cseh K, Baranyi É, Melczer Z, Kaszás E, Palik É, Winkler, G. Plasma adiponectin and pregnancy-induced insulin resistance. Diabet Care. 2004;27:274–5.
Kuzmicki M, Telejko B, Szamatowicz J, Zonenberg A, Nikolajuk A, Kretowski A, et al. High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecol Endocrinol. 2009;25:258–63.
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12.
Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, Considine RV, et al. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-γ action in humans. Diabetes. 2001;50:2199–202.
Janke J, Engeli S, Gorzelniak K, Luft FC, Sharma AM. Resistin gene expression in human adipocytes is not related to insulin resistance. Obes Res. 2002;10:1–5.
Yura S, Sagawa N, Itoh H, Kakui K, Nuamah MA, Korita D, et al. Resistin is expressed in the human placenta. J Clin Endocrinol Metab. 2003;88:1394–7.
Nien JK, Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, et al. Resistin: a hormone which induces insulin resistance is increased in normal pregnancy. J Perinat Med. 2007;35:513–21.
McTernan PG, Fisher FM, Valsamakis G, Chetty R, Harte A, McTernan CL, et al. Resistin and type 2 diabetes: regulation of resistin expression by insulin and rosiglitazone and the effects of recombinant resistin on lipid and glucose metabolism in human differentiated adipocytes. J Clin Endocrinol Metab. 2003;88:6098–106.
Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than NG, et al. Maternal visfatin concentration in normal pregnancy. J Perinat Med. 2009;37:206–17.
Telejko B, Kuzmicki M, Zonenberg A, Szamatowicz J, Wawrusiewicz-Kurylonek N, Nikolajuk A, et al. The role of adipocytokines in insulin resistance in normal pregnancy: visfatin concentrations in early pregnancy predict insulin sensitivity. Clin Chem. 2007;53:1477–83.
Telejko B, Kuzmicki M, Zonenberg A, Szamatowicz J, Wawrusiewicz-Kurylonek N, Nikolajuk A, et al. Visfatin in gestational diabetes: serum level and mRNA expression in fat and placental tissue. Diabetes Res Clin Pract. 2009;84:68–75.
Morgan SA, Bringolf JB, Seidel ER. Visfatin expression is elevated in normal human pregnancy. Peptides. 2008;29:1382–9.
Hossein-nezhad A, Mirzaei K, Maghbooli Z, Rahmani M, Larijani B. Resistin, adiponectin and visfatin; can adipocytokines predict gestational diabetes mellitus and early post partum metabolic syndrome? Iran J Diabetes Lipid Dis. 2010;9:1–8.
Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, Shnawa N, et al. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci. 2006;110:605–9.
Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–17.
Li L, Yang G, Li Q, Tang Y, Yang M, Yang H, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006;114:544–8.
Chan TF, Chen YL, Lee CH, Chou FH, Wu LC, Jong SB, et al. Decreased plasma visfatin concentrations in women with gestational diabetes mellitus. J Soc Gynecol Investig. 2006;13:364–7.
Berndt J, Klöting N, Kralisch S, Kovacs P, Fasshauer M, Schön MR, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–6.
Winkler G, Cseh K, Baranyi E, Melczer Z, Speer G, Hajós P, et al. Tumor necrosis factor system in insulin resistance in gestational diabetes. Diabetes Res Clin Pract. 2002;56:93–9.
Sternberg EM, Chrousos GP, Wilder RL, Gold PW. The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992;117:854–66.
Coughlan MT, Oliva K, Georgiou HM, Permezel JMH, Rice GE. Glucose-induced release of tumor necrosis factor-alpha from human placental and adipose tissues in gestational diabetes mellitus. Diabet Med. 2001;18:921–7.
Acknowledgments
The authors are grateful to the Deanship of Scientific Research, King Abdul Aziz University, Jeddah, Saudi Arabia, for the generous financial support of the Project No. 17-026/430. The authors also thank Prof. Hassan S Abduljabbar, Consultant and Professor of Obstetrics and Gynecology, King Abdul Aziz University Hospital and Dr. Nabeela Y. Qusti, Senior Specialist in Maternity and Children Hospital, Jeddah, for their assistance in diagnosing and collecting samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noureldeen, A.F.H., Qusti, S.Y., Al-seeni, M.N. et al. Maternal Leptin, Adiponectin, Resistin, Visfatin and Tumor Necrosis Factor-Alpha in Normal and Gestational Diabetes. Ind J Clin Biochem 29, 462–470 (2014). https://doi.org/10.1007/s12291-013-0394-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-013-0394-0