Abstract
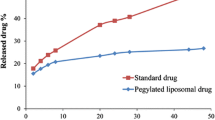
This study is aimed to investigate the nanoliposomal artemisinin preparation, and its implementation on breast cancer cells. Side effects have been one of the common challenges of drug usage, as well as cancer treatment. In order to reduce such effects, nanotechnology has been a great help. Nanoliposomes are provided through reverse phase evaporation. In this method, certain proportions of phosphatidylcholine, cholesterol and artemisinin were mixed together. Besides, the obtained formulation was pegylated by using polyethylene glycol 2000 in order to increase its stability and solubility. The mean diameter of non-pegylated and pegylated liposomal artemisinin was determined by Zeta sizer system. The percent of drug released from liposome was performed by dialysis. The encapsulation efficiency of both formulations was estimated by spectrophotometry method. As a result, encapsulation and drug release of nanoliposomal formulation were more than the pegylation of the same formulation. In addition, this study indicated that cytotoxicity effect of pegylated nanoliposomal artemisinin was more, in comparison with nanoliposomal artemisinin.



Similar content being viewed by others
References
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60.
Warner E. Clinical practice. Breast-cancer screening. N Engl J Med. 2011;365:1025–32.
Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–9.
Mohammed AR, Weston N, Coombes AG, Fitzgerald M, Perrie Y. Liposome formulation of poorly water soluble drugs: optimisation of drug loading and ESEM analysis of stability. Int J Pharm. 2004;285:23–34.
Wagner A, Vorauer-Uhl K. Liposome technology for industrial purposes. J Drug Deliv. 2011;2011:591325.
Lia T, Ho R. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90:667–80.
Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354:28–33.
Lai H, Singh NP. Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 2006;231:43–8.
Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf. 2004;27:25–61.
Leonardi E, Gilvary G, White NJ, Nosten F. Severe allergic reactions to oral artesunate: a report of two cases. Trans R Soc Trop Med Hyg. 2001;95:182–3.
Zhang Z, Feng SS. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials. 2006;27:4025–33.
Chadwick J, Mercer AE, Park BK, Cosstick R, O’Neill PM. Synthesis and biological evaluation of extraordinarily potent C-10 carba artemisinin dimers against P. falciparum malaria parasites and HL-60 cancer cells. Bioorg Med Chem. 2009;17:1325–38.
Chen H, Sun B, Pan S, Jiang H, Sun X. Dihydroartemisinin inhibits growth of pancreatic cancer cells in vitro and in vivo. Anticancer Drugs. 2009;20:131–40.
Lai HC, Singh NP, Sasaki T. Development of artemisinin compounds for cancer treatment. Investig New Drugs. 2013;31:230–46.
Ning Z, Cheung CS, Fu J, Liu MA, Schnell MA. Experimental study of environmental tobacco smoke particles under actual indoor environment. Sci Total Environ. 2006;367:822–30.
Yatuv R, Robinson M, Dayan-Tarshish I, Baru M. The use of PEGylated liposomes in the development of drug delivery applications for the treatment of hemophilia. Int J Nanomed. 2010;5:581–91.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dadgar, N., Koohi Moftakhari Esfahani, M., Torabi, S. et al. Effects of Nanoliposomal and Pegylated Nanoliposomal Artemisinin in Treatment of Breast Cancer. Ind J Clin Biochem 29, 501–504 (2014). https://doi.org/10.1007/s12291-013-0389-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-013-0389-x