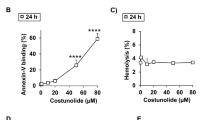
Abstract
Malaria infection is known to cause severe hemolysis due to production of abnormal RBCs and enhanced RBC destruction through apoptosis. Infected RBC lysis exposes uninfected RBC to the large amount of pro-oxidant molecules such as methemoglobin. Methemoglobin (MetHb) exposure dose dependently makes RBCs susceptible to osmotic stress and causes hemolysis. MetHb mediated oxidative stress in RBC correlated well with osmotic fragility and hemolysis. Interestingly, a reactive oxygen species (ROS) spike at 15 min was responsible for the observed effects on RBC cells. Two natural antioxidants N-acetyl cysteine and mannitol protected the RBC from MetHb-mediated defects, which clearly indicated involvement of oxidative stress in the process. MetHb due to its pseudo-peroxidase activity produces ROS in the external microenvironment. Therefore, classical peroxidase inhibitors were tested to probe peroxidase activity mediated ROS production with defects in RBCs. Clotrimazole (CLT), which irreversibly inactivates the MetHb (CLT-MetHb) and abolishes peroxidase activity, did not produce significant ROS outside RBC and was inefficient to cause osmotic fragility and hemolysis. Hence, initiating a chain reaction, MetHb released from ruptured RBC produces significant ROS in the external microenvironment to make RBC membrane leaky and enhanced hemolysis. Together data presented in the current work explored the role of MetHb in accelerated humorless during malaria which could be responsible for severe outcomes of pathological disorders.





Similar content being viewed by others
Abbreviations
- GSH:
-
Reduced glutathione
- NAC:
-
N-acetyl cysteine
- ROS:
-
Reactive oxygen species
- MetHb:
-
Methemoglobin
- CLT-MetHb:
-
Clotrimazole modified methemoglobin
References
McQueen PG, McKenzie FE. Age-structured red blood cell susceptibility and the dynamics of malaria infections. Proc Natl Acad Sci USA. 2004;101:9161–6.
Condon MR, Kim JE, Deitch EA, Machiedo GW, Spolarics Z. Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am J Physiol Heart Circ Physiol. 2003;284:H2177–84.
Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–62.
Pamplona A, Hanscheid T, Epiphanio S, Mota MM, Vigario AM. Cerebral malaria and the hemolysis/methemoglobin/heme hypothesis: shedding new light on an old disease. Int J Biochem Cell Biol. 2009;41:711–6.
Totino PR, Magalhaes AD, Silva LA, Banic DM, Daniel-Ribeiro CT, Ferreira-da-Cruz Mde F. Apoptosis of non-parasitized red blood cells in malaria: a putative mechanism involved in the pathogenesis of anaemia. Malar J. 2010;9:350.
Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7:113–6.
Balla J, Jacob HS, Balla G, Nath K, Eaton JW, Vercellotti GM. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci USA. 1993;90:9285–9.
Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646–56.
Matarrese P, Straface E, Pietraforte D, et al. Peroxynitrite induces senescence and apoptosis of red blood cells through the activation of aspartyl and cysteinyl proteases. FASEB J. 2005;19:416–8.
Boretti FS, Buehler PW, D’Agnillo F, et al. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119:2271–80.
Goldman DW, Breyer RJ 3rd, Yeh D, Brockner-Ryan BA, Alayash AI. Acellular hemoglobin-mediated oxidative stress toward endothelium: a role for ferryl iron. Am J Physiol. 1998;275:H1046–53.
Buehler PW, D’Agnillo F. Toxicological consequences of extracellular hemoglobin: biochemical and physiological perspectives. Antioxid Redox Signal. 2010;12:275–91.
Trivedi V, Chand P, Srivastava K, Puri SK, Maulik PR, Bandyopadhyay U. Clotrimazole inhibits hemoperoxidase of Plasmodium falciparum and induces oxidative stress. Proposed antimalarial mechanism of clotrimazole. J Biol Chem. 2005;280:41129–36.
Trivedi V, Srivastava K, Puri SK, Maulik PR, Bandyopadhyay U. Purification and biochemical characterization of a heme containing peroxidase from the human parasite P. falciparum. Protein Expr Purif. 2005;41:154–61.
Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55.
Reeder BJ, Svistunenko DA, Cooper CE, Wilson MT. The radical and redox chemistry of myoglobin and hemoglobin: from in vitro studies to human pathology. Antioxid Redox Signal. 2004;6:954–66.
Widmer CC, Pereira CP, Gehrig P, et al. Hemoglobin can attenuate hydrogen peroxide-induced oxidative stress by acting as an antioxidative peroxidase. Antioxid Redox Signal. 2010;12:185–98.
Wagner MA, Andemariam B, Desai SA. A two-compartment model of osmotic lysis in Plasmodium falciparum-infected erythrocytes. Biophys J. 2003;84:116–23.
Beck JS, Saari JT. Permeability coefficients by the hemolytic method: a correction. Biophys J. 1977;17:281–2.
Brumen M, Heinrich R. A metabolic osmotic model of human erythrocytes. Biosystems. 1984;17:155–69.
Hoshen MB, Heinrich R, Stein WD, Ginsburg H. Mathematical modelling of the within-host dynamics of Plasmodium falciparum. Parasitology. 2000;121(Pt 3):227–35.
Lisovskaia IL, Ataullakhanov FI, Tuzhilova EG, Vitvitskii VM. Analysis of geometric parameters and mechanical properties of erythrocytes by filtration through nuclear membrane filters. II. Experimental verification of a mathematical model. Biofizika. 1994;39:864–71.
Lamy ML, Daily EK, Brichant JF, et al. Randomized trial of diaspirin cross-linked hemoglobin solution as an alternative to blood transfusion after cardiac surgery. The DCLHb Cardiac Surgery Trial Collaborative Group. Anesthesiology. 2000;92:646–56.
Przybelski RJ, Daily EK, Micheels J, et al. A safety assessment of diaspirin cross-linked hemoglobin (DCLHb) in the treatment of hemorrhagic, hypovolemic shock. Prehosp Disaster Med. 1999;14:251–64.
Sloan EP, Koenigsberg M, Gens D, et al. Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock: a randomized controlled efficacy trial. JAMA. 1999;282:1857–64.
Hogman CF, Eriksson L, Ericson A, Reppucci AJ. Storage of saline-adenine-glucose-mannitol-suspended red cells in a new plastic container: polyvinylchloride plasticized with butyryl-n-trihexyl-citrate. Transfusion. 1991;31:26–9.
Scott KL, Lecak J, Acker JP. Biopreservation of red blood cells: past, present, and future. Transfus Med Rev. 2005;19:127–42.
Acknowledgments
This work has partially been supported by the Department of Atomic energy (DAE) young scientist award and IITG startup grant to VT. Both Authors acknowledge the infrastructure facility provided by the Department of Biotechnology, IIT-Guwahati, Guwahati, Assam (India). We are thankful to Dr. Amogh A Sahasrabuddhe, Scientist, Central Drug Research Institute (CDRI), Lucknow for valuable suggestions and for critically reviewing the manuscript.
Conflict of interest
No competing financial interests exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balaji, S.N., Trivedi, V. Extracellular Methemoglobin Mediated Early ROS Spike Triggers Osmotic Fragility and RBC Destruction: An Insight into the Enhanced Hemolysis During Malaria. Ind J Clin Biochem 27, 178–185 (2012). https://doi.org/10.1007/s12291-011-0176-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-011-0176-5