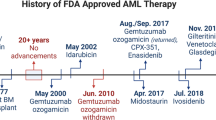
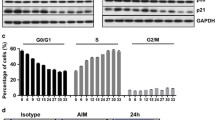
Abstract
Acute lymphoblastic leukemia (ALL) is a malignant disease characterized by an uncontrolled proliferation of immature lymphoid cells. ALL is the most common hematologic malignancy in early childhood, and it reaches peak incidence between the ages of 2 and 3 years. The prognosis of ALL is associated with aberrant gene expression, in addition to the presence of numerical or structural chromosomal alterations, age, race, and immunophenotype. The Relapse rate with regard to pharmacological treatment rises in childhood; thus, the expression of biomarkers associated with the activation of cell signaling pathways is crucial to establish the disease prognosis. Intracellular pathways involved in ALL are diverse, including Janus kinase/Signal transducers and transcription activators (JAK-STAT), Phosphoinositide-3-kinase–protein kinase B (PI3K-AKT), Ras mitogen-activated protein kinase (Ras-MAPK), Glycogen synthase kinase-3β (GSK-3β), Nuclear factor-kappa beta (NF-κB), and Hypoxia-inducible transcription factor 1α (HIF-1α), among others. In this review, we present several therapeutic targets, intracellular pathways, and molecular markers that are being studied extensively at present.
Similar content being viewed by others
References
Silverman LB (2014) Balancing cure and long-term risks in acute lymphoblastic leukemia. Hematology 25(1):190–197
Campbell K, Gerscher S, Siclair L (2001) Childhood acute lymphoblastic leukaemia. Leukaemia Research Fund 8(1):1–10
Pacheco C, Lucchini G, Valsecchi MG, Malta A, Conter V, Flores A et al (2014) Childhood acute lymphoblastic leukemia in Nicaragua: long-term results in the context of an international cooperative program. Pediatr Blood Cancer 61(5):827–832
Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P et al (1996) Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol 14(1):18–24
Harrison CJ (2013) Targeting signaling pathways in acute lymphoblastic leukemia: new insights. ASH Educ Book 1:118–125
Friedmann AM, Weinstein HJ (2000) The role of prognostic features in the treatment of childhood acute lymphoblastic leukemia. Oncologist 5:321–328
Winter SS (2011) Pediatric acute leukemia therapies informed by molecular analysis of high-risk disease. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology Education Program, pp 366–73
Protocolo de la atención para leucemia linfoblástica. guía clínica y esquema de tratamiento. Instituto nacional de salud pública, validado por el consejo de salubridad general, los institutos nacionales de salud y la comisión nacional de protección social en salud. Seguro Popular 1(1):1–20
Wheeler KA, Richards SM, Bailey CC, Gibson B, Hann IM, Hill FG et al (2000) Bone marrow transplantation versus chemotherapy in the treatment of very high-risk childhood acute lymphoblastic leukemia in first remission: results from Medical Research Council UK ALL X and XI. Blood 96:2412–2451
Waber DP, Carpentieri SC, Klar N, Silverman LB, Schwenn M, Hurwitz CA et al (2000) Cognitive sequelae in children treated for acute lymphoblastic leukemia with dexamethasone or prednisone. J Pediatr Hematol Oncol 22:206–213
Cao Y, Lupo PJ, Swartz MD, Nousome D, Scheurer ME (2013) Using a bayesian hierarchical model for identifying single nucleotide polymorphisms associated with childhood acute lymphoblastic leukemia risk in case-parent triads. PLoS One 8(12):1–5
Aziz SA, Sharma SK, Sabah I, Jan MA (2015) Prognostic significance of cell surface phenotype in acute lymphoblastic leukemia. South Asian J Cancer 4(2):91–94
Ross ME, Mahfouz R, Onciu M, Liu HC, Zhou X, Song G et al (2004) Gene expression profiling of pediatric acute myelogenous leukemia. Blood 104:3679–3687
Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL et al (2008) Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood 111:5477–5485
Roganovic J, Guenova M, Fuchs O, Abdul G, Schuurhuis GJ, Zeijlemaker W et.al. Leukemia, 1ra Ed. ISBN 978-953-51-1127-6
Linka Y, Ginzel S, Krüger M, Novosel A, Gombert M, Kremmer E et al. (2013) The impact of TEL-AML1 (ETV6-RUNX1) expression in precursor B cells and implications for leukaemia using three different genome-wide screening methods. Blood Cancer J 11(3):151
Wang J, Mi JQ, Debernardi A, Vitte AL, Emadali A, Meyer JA et.al. (2015) A six gene expression signature defines aggressive subtypes and predicts outcome in childhood and adult acute lymphoblastic leukemia. Oncotarget 6(18):16527–16542
Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA et al (2008) Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 322:1377–1380
Cario G, Zimmermann M, Romey R, Gesk S, Vater I, Harbott J et al (2010) Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prognosis in non-high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood 115:5393–5397
Bercovich D, Ganmore I, Scott LM, Wainreb G, Birger Y, Elimelech A et al (2008) Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down’s syndrome. Lancet 372:1484–1492
Ward A, Touw I, Yoshimura A (2000) The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood 95:19–29
Mullighan C, Zhang J, Harvey R, Collins-Underwood J, Schulman B, Phillips L et al (2009) JAK mutations in highrisk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci USA 9(106):9414–9418
Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD et al (1998) Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-in- duced biologic responses. Cell 93:373–383
Darnell JE, Kerr IM, Stark GR (1994) Jak-STAT path- ways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415–1421
Ihle JN, Witthuhn BA, Quelle FW, Yamamoto K, Silvennoinen O (1995) Signaling through the hematopoietic cytokine receptors. Annu Rev Immunol 13:369–398
O’Shea JJ, Leonard WJ (1995) Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270:797–800
Lo MC, Peterson LF, Yan M, Cong X, Hickman JH, Dekelver RC et al (2013) JAK inhibitors suppress t(8;21) fusion protein-induced leukemia. Leukemia 27:2272–2279
Mullighan C (2012) The molecular genetic makeup of acute lymphoblastic leukemia, 1st edn. ASH Educ Book 1:389–396
Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S et al (1998) Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93:385–395
Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K (1998) Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 93:397–409
Silvennoinen O, Witthuhn B, Quelle FW, Cleveland JL, Yi T, Ihle JN (1993) Structure of the JAK2 protein tyrosine kinase and its role in IL-3 signal transduction. Proc Natl Acad Sci 90:8429–8433
Yeh TC, Pellegrini S (1999) The Janus kinase family of protein tyrosine kinases and their role in signaling. Cell Mol Life Sci 55:1523–1534
Constantinescu SN, Ghaffari S, Lodish HF (1999) The erythropoietin receptor: structure, activation, and intracellular signal transduction. Trends Endocrinol 10:18–23
Verstovsek S, Passamonti F, Rambaldi A, Barosi G, Rosen PJ, Rumi E et al (2013) A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer 120:513–520
Patterer V, Schnittger S, Kern W, Haferlach T, Haferlach C (2013) Hematologic malignancies with PCM1-JAK2 gene fusion share characteristics with myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB, and FGFR1. Ann Hematol 92:759–769
Yu V, Pistillo J, Archibeque I, Han Lee J, Sun BC, Schenkel LB et al (2013) Differential selectivity of JAK2 inhibitors in enzymatic and cellular settings. Exp Hematol 41:491–500
Ghaffari S, Kitidis C, Fleming M, Neubauer H, Pfeffer K, Lodish H (2001) Erythropoiesis in the absence of januskinase 2: BCR-ABL induces red cell formation in JAK2−/− hematopoietic progenitors. Blood 98:2948–2957
Nosaka T, van Deursen JM, Tripp RA, Thierfelder WE, Witthuhn BA, McMickle AP et al (1995) Defectivelymphoid development in mice lacking Jak3. Science 270:800–802
Grossman WJ, Verbsky JW, Yang L, Berg LJ, Fields LE, Chaplin DD et al (1999) Dysregulated myelopoiesis in mice lacking Jak3. Blood 94:932–939
Warsi J, Hosseinzadeh Z, Dong L, Pakladok T, Umbach A, Bhavsar S et al (2013) Effect of Janus kinase 3 on the peptide transporters PEPT1 and PEPT2. J Membr Biol 246:885–892
Ross JA, Spadaro M, Rosado DC, Cavallo F, Kirken RA, Pericle F (2013) Inhibition of JAK3 with a novel, selective and orally active small molecule induces therapeutic response in T-cell malignancies. Leukemia 28(4):941–944
Zenatti PP, Ribeiro D, Li W, Zuurbier L, Silva MC, Paganin M et al (2011) Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat Genet 43:932–939
Ott CJ, Kopp N, Bird L, Paranal RM, Qi J, Bowman T (2012) BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood 120:2843–2852
Pardanani A, Finke C, Lasho TL, Al-Kali A, Begna KH, Hanson C et al (2012) IPSS-independent prognostic value of plasma CXCL10, IL-7 and IL-6 levels in myelodysplastic síndromes. Leukemia 26:693–699
Kornblau SM, McCue D, Singh N, Chen W, Estrov Z, Coombes KR (2010) Recurrent expression signatures of cytokines and chemokines are present and are independently prognostic in acute myelogenous leukemia and myelodysplasia. Blood 116:4251–4261
Sanda T, Tyner JW, Gutierrez A, Ngo VN, Glover J, Chang BH et al (2013) TYK2-STAT1-BCL2 pathway dependence in T-cell acute lymphoblastic leukemia. Cancer Discov 3:564–577
Kirito K, Nakajima K, Watanabe T, Uchida M, Tanaka M, Ozawa K et al (2002) Identification of the human erythropoietin receptor region required for Stat1 and Stat3 activation. Blood 99:102–110
Walker S, Frank DA (2012) Screening approaches to generating STAT inhibitors: allowing the hits to identify the targets. JAK-STAT 1(4):292–299
Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB et al (2011) The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood 117:3421–3429
Coppo P, Flamant S, De Mas V, Jarrier P, Guillier M, Bonnet ML et al (2006) BCR–ABL activates STAT3 via JAK and MEK pathways in human cells. Br J Haematol 134:171–179
Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S (1996) Essential role of Stat6 in IL-4 signalling. Nature 380:627–630
Kindler T, Cornejo M, Scholl C, Liu J, Leeman D, Haydu J et al (2008) K-RasG12D–induced T-cell lymphoblastic lymphoma/leukemias harbor Notch1 mutations and are sensitive to γ-secretase inhibitors. Blood 112:3373–3382
Lee-Sherick AB, Linger RM, Gore L, Keating AK, Graham DK (2010) Targeting paediatric acute lymphoblastic leukaemia: novel therapies currently in development. Br J Haematol 151:295–311
Hidalgo M, Rowinsky EK (2000) The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene 19:6680–6686
Avellino R, Romano S, Parasole R, Bisogni R, Lamberti A, Poggi V et al (2005) Rapamycin stimulates apoptosis of childhood acute lymphoblastic leukemia cells. Blood 106:1400–1406
Nyga R, Pecquet C, Harir N, Gu H, Dhennin-Duthille I, Regnier A et al (2005) Activated STAT5 proteins induce activation of the PI 3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter. Biochem J 390:359–366
Badura S, Tesanovic T, Pfeifer H, Wystub S, Nijmeijer B, Liebermann M et.al. (2013) Differential effects of selective inhibitors targeting the PI3K/AKT/mTOR pathway in acute lymphoblastic leukemia. PLoS One 8(11):e80070
Hu Y, Gu X, Li R, Luo Q, Xu Y (2010) Glycogen synthase kinase-3 beta inhibition induces nuclear factor-kappaBmediated apoptosis in pediatric acute lymphocyte leukemia cells. J Exp Clin Cancer Res 29:154
Ding VW, Chen RH, McCormick F (2000) Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem 275:32475–32481
Shin S, Wolgamott L, Yu Y, Blenis J, Yoon SO (2011) Glycogen synthase kinase (GSK)-3 promotes p70 ribosomal protein S6 kinase (p70S6K) activity and cell proliferation. Proc Natl Acad Sci USA 108:1204–1213
Dann SG, Selvaraj A, Thomas G (2007) mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med 13:252–259
Carnevalli LS, Masuda K, Frigerio F, Le Bacquer O, Um SH, Gandin V et al (2010) S6K1 plays a critical role in early adipocyte differentiation. Dev Cell 18:763–774
Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL et al (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39:171–183
Flügel D (2012) Görlach, Kietzmann T. GSK-3β regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1. Blood 119:1292–1301
Torrano V, Procter J, Cardus P, Greaves M, Ford A (2011) ETV6-RUNX1 promotes survival of early B lineage progenitor cells via a dysregulated erythropoietin receptor. Blood 118:4910–4918
Dowdyb C, Frederickb D, Zaidia S, Colbyb J, Liana J, Van Wijnenc J et al (2013) A germline point mutation in Runx1 uncouples its role in definitive hematopoiesis from differentiation. Exp Hematol 41:980–991
Goyama S, Schibler J, Cunningham L, Zhang Y, Rao Y, Nishimoto N et al (2013) Transcription factor RUNX1 promotes survival of acute myeloid leukemia cells. J Clin Invest 123(9):3876–3888
Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Christopher B et al (2009) Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med 360:470–480
Starkova J, Zamostna B, Mejstrikova E, Krejci R, Drabkin HA, Trka J (2010) HOX gene expression in phenotypic and genotypic subgroups and low HOXA gene expression as an adverse prognostic factor in pediatric ALL. Pediatr Blood Cancer 55:1072–1082
Beachy SH, Onozawa M, Silverman D, Chung YJ, Rivera MM, Aplan PD (2013) Isolated Hoxa9 overexpression predisposes to the development of lymphoid but not myeloid leukemia. Exp Hematol 41:518–529
Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC et al (2002) Gene expression signatures define novel onco- genic pathways in T cell acute lymphoblastic leukemia. Cancer Cell 1:75–87
De Keersmaecker K, Marynen P, Cools J (2005) Genetic insights in the pathogenesis of T-cell acute lymphoblastic leukemia. Haematologica 90:1116–1127
Homminga I, Vuerhard MJ, Langerak AW, Buijs-Gladdines J, Pieters R, Meijerink JP (2012) Characterization of a pediatric T-cell acute lymphoblastic leukemia patient with simultaneous LYL1 and LMO2 rearrangements. Haematologica 97:258–261
Tatarek J, Cullion K, Ashworth T, Gerstein R, Aster JC, Kelliher MA (2011) Notch1 inhibition targets the leukemia initiating cells in a Tal1/Lmo2 mouse model of T-ALL. Blood 118(6):1579–1590
Kusy S, Gerby B, Goardon N, Gault N, Ferri F, Gérard D et al (2010) NKX3.1 is a direct TAL1 target gene that mediates proliferation of TAL1-expressing human T cell acute lymphoblastic leukemia. J Exp Med 207:2141–2156
Wadman I, Li J, Bash RO, Forster A, Osada H, Rabbitts TH et al (1994) Specific in vivo association between the bHLH and LIM proteins implicated in human T cell leukemia. EMBO J 13:4831–4839
Baer R (1993) TAL1, TAL2 and LYL1: a family of basic helix-loop-helix proteins implicated in T cell acute leukaemia. Semin Cancer Biol 4:341–347
Martin GS (2001) The hunting of the Src. Nat Rev Mol Cell Biol 2:467–475
Venkitachalam S, Chueh FY, Yu CL (2012) Nuclear localization of lymphocyte-specific protein tyrosine kinase (Lck) and its role in regulating LIM domain only 2 (Lmo2) gene. Biochem Biophys Res Commun 417:1058–1062
Miyamoto A, Cui X, Naumovski L, Cleary ML (1996) Helix-loop-helix proteins LYL1 and E2a form heterodimeric complexes with distinctive DNA-binding properties in hematolymphoid cells. Mol Cell Biol 16(5):2394–2401
Pui CH, Carroll WL, Meshinchi S, Arceci RJ (2011) Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol 29:551–565
Armstrong SA, Look AT (2005) Molecular genetics of acute lymphoblastic leukemia. J Clin Oncol 23:6306–6315
García JL, Hernández JM, Gutiérrez NC, Flores T, González D, Calasanz MJ et al (2003) Abnormalities on 1q and 7q are associated with poor outcome in sporadic Burkitt’s lymphoma. A cytogenetic and comparative genomic hybridization study. Leukemia 17:2016–2024
Zunino A, Viaggi S, Ottaggio L, Fronza G, Schenone A, Roncella S et al (2000) Chromosomal aberrations evaluated by CGH, FISH and GTG-banding in a case of AIDS-related Burkitt’s lymphoma. Haematologica 85:250–255
Zimonjic DB, Keck-Waggoner C, Popescu NC (2001) Novel genomic imbalances and chromosome translocations involving c-myc gene in Burkitt’s lymphoma. Leukemia 15:1582–1588
Salaverria I, Zettl A, Beà S, Hartmann EM, Dave SS, Wright GW et al (2008) Chromosomal alterations detected by comparative genomic hybridization in subgroups of gene expression-defined Burkitt’s lymphoma. Haematologica 93:1327–1334
Barth TF, Müller S, Pawlita M, Siebert R, Rother JU, Mechtersheimer G et al (2004) Homogeneous immunophenotype and paucity of secondary genomic aberrations are distinctive features of endemic but not of sporadic Burkitt’s lymphoma and diffuse large B-cell lymphoma with MYC rearrangement. J Pathol 203:940–945
Toujani S, Dessen P, Ithzar N, Danglot G, Richon C, Vassetzky Y et al (2009) High resolution genome-wide analysis of chromosomal alterations in Burkitt’s lymphoma. PLoS One 4:7089
Hawley RG, Fong AZ, Reis MD, Zhang N, Lu M, Hawley TS (1997) Transforming function of the HOX11/TCL3 homeobox gene. Cancer Res 57:337–345
Keller G, Wall C, Fong AZ, Hawley TS, Hawley RG (1998) Overexpression of HOX11 leads to the immortalization of embryonic precursors with both primitive and definitive hematopoietic potential. Blood 92:877–887
De Keersmaecker K, Marynen P, Cools J (2005) Genetic insights in the pathogenesis of T-cell acute lymphoblastic leukemia. Haematologica 90:1116–1127
Uckun FM, Myers DE, Qazi S, Ozer Z, Rose R, D’Cruz OJ et al (2015) Recombinant human CD19L-sTRAIL effectively targets B cell precursor acute lymphoblastic leukemia. J Clin Investig 125(3):1006–1018
Hasan M, Queudeville M, Trentin L, Mirjam S, Bronzini I, Palmi C et al (2014) Targeting of hyperactivated mTOR signaling in high-risk acute lymphoblastic leukemia in a pre-clinical model. Oncotarget 6(3):1382–1395
Beagle BR, Nguyen DM, Mallya S, Tang SS, Lu M, Zeng Z et al (2015) mTOR kinase inhibitors synergize with histone deacetylase inhibitors to kill B-cell acute lymphoblastic leukemia cells. Oncotarget 6(4):2088–2100
Evangelisti C, Evangelisti C, Teti G, Chiarini F, Falconi M, Melchionda F et al (2014) Assessment of the effect of sphingosine kinase inhibitors on apoptosis, unfolded protein response and autophagy of T-cell acute lymphoblastic leukemia cells; indications for novel therapeutics. Oncotarget 5(17):7886–7901
Ntziachristos P, Tsirigos A, Welstead G, Trimarchi T, Bakogianni S, Xu L et al (2014) Contrasting roles for histone 3 lysine 27 demethylases in acute lymphoblastic leukemia. Nature 514(7523):513–517
Hu Z, Slayton WB (2014) Integrin VLA-5 and FAK are good targets to improve treatment response in the philadelphia chromosome positive acute lymphoblastic leukemia. Front Oncol 4:112
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Layton Tovar, C.F., Mendieta Zerón, H. Intracellular Signaling Pathways Involved in Childhood Acute Lymphoblastic Leukemia; Molecular Targets. Indian J Hematol Blood Transfus 32, 141–153 (2016). https://doi.org/10.1007/s12288-015-0609-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-015-0609-z