Abstract
Background
In breast cancer patients receiving neoadjuvant chemotherapy (NAC), immediate breast reconstruction (IBR) as a breast cancer treatment option remains controversial. We assessed the impact of NAC on surgical and oncological outcomes of patients undergoing IBR.
Methods
This was a retrospective multicenter study of 4726 breast cancer cases undergoing IBR. The rate of postoperative complications and survival data were compared between IBR patients who received NAC and those who did not receive NAC. Propensity score matching analysis was performed to mitigate selection bias for survival.
Results
Of the total 4726 cases, 473 (10.0%) received NAC. Out of the cases with NAC, 96 (20.3%) experienced postoperative complications, while 744 cases (17.5%) without NAC had postoperative complications. NAC did not significant increase the risk of complications after IBR (Odds ratio, 0.96; 95%CI 0.74–1.25). At the median follow-up time of 76.5 months, 36 patients in the NAC group and 147 patients in the control group developed local recurrences. The 5-year local recurrence-free survival rate was 93.1% in the NAC group and 97.1% in the control group. (P < 0.001). After matching, there was no significant difference between the two groups.
Conclusion
IBR after NAC is a safe procedure with an acceptable postoperative complication profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most prevalent malignancy affecting women worldwide, including Japanese women [1, 2]. In recent years, neoadjuvant chemotherapy (NAC) has gained widespread use in early-stage breast cancer patients. Initially, NAC was employed to convert inoperable locally advanced breast cancers into operable tumors and to downstage such tumors allowing breast-conserving surgery [3, 4]. However, NAC is now recognized for its role in improving outcomes through residual disease-guided approach [5]. Patients with residual tumors after NAC have various indications for adjuvant therapies such as trastuzumab emtansine for human epidermal growth factor receptor-2 (HER2) -positive tumors, capecitabine for triple-negative tumors, Poly (ADP-ribose) polymerase inhibitors for those with BRCA pathogenic variants, cyclin-dependent kinase 4/6 inhibitors, and S-1 for hormone receptor-positive, HER2-negative tumors [6,7,8,9,10].
As another comprehensive component of breast cancer care, immediate breast reconstruction (IBR) after mastectomy increased with the establishment of IBR procedures. IBR helps restore body image and has a positive impact on psychological well-being and quality of life for patients [11,12,13]. Moreover, the use of nipple-sparing mastectomy (NSM) and skin-sparing mastectomy (SSM) procedures have increased, leading to improved aesthetic outcomes. Therefore, IBR with NSM/SSM techniques has emerged as an important surgical option after NAC [14]. Despite the advantages of IBR, there is a persistent concern among physicians that IBR after NAC may delay adjuvant therapy due to postoperative complications and thereby increase the risk of locoregional recurrence [15,16,17,18]. Additionally, post-mastectomy radiation therapy (PMRT) is reportedly associated with a higher incidence of adverse cosmetic outcomes in patients undergoing IBR [19, 20].
Several studies have shown that IBR after NAC does not significantly increase complications [21,22,23,24,25,26], and previous studies have reported the prognosis of breast cancer patients with IBR after NAC is comparable to those with mastectomy alone after NAC [14, 27, 28], but it is still under debate. Therefore, we conducted a multicenter, retrospective, observational study in collaboration with the Study Group of Scientific Research of the Japan Breast Cancer Society. We aimed to investigate the potential impact of NAC on postoperative complications and locoregional recurrence in breast cancer patients undergoing IBR.
Patients and methods
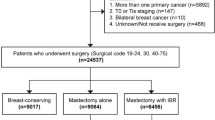
We conducted a retrospective review of the medical records of 4726 consecutive breast cancer patients who underwent IBR between January 2008 and December 2016. The data were collected from 12 institutes and included various clinicopathological factors such as menopause status, body mass index (BMI), smoking status, pathological tumor size, nodal status, estrogen receptor (ER) expression, progesterone receptor (PgR) expression, and HER2 expression, the presence of lymphovascular invasion, and surgical margin status. Information on treatment modalities, including surgical technique, reconstruction type, axillary surgery, adjuvant therapy, NAC, and PMRT was also collected. Furthermore, data on wound complications, including infection, hemorrhage, seroma, dehiscence, flap necrosis, seroma, marginal necrosis, nipple necrosis respectively after initial surgery, capsular contracture, malrotation, rupture and loss of prosthesis and loss of total flap as well as oncologic outcomes including local and regional recurrence, were examined.
This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 1983, and was approved by the Ethics Committee of each participating institute. Due to the retrospective nature of the study, the requirement for informed consent from the patients was waived.
ER and PgR expressions were assessed using immunohistochemistry, and tumors in which 1% or more of tumor cells stained positive were classified as ER or PgR positive. HER2 status was considered positive if the immunohistochemistry result was 3 + or confirmed by fluorescence in situ hybridization with an amplification ratio of ≥ 2.0. The evaluation of ER, PgR, and HER2 statuses was performed by each participating institution. BMI was classified as normal (BMI 18–24) or overweight (BMI ≥ 25). We divided postoperative complications according to the Clavien-Dindo classification. Local recurrence was defined as the presence of any breast cancer in the ipsilateral breast, skin, subcutaneous tissue, pectoralis muscle, or thoracic wall. Regional recurrence was defined as metastases involving the axillary, supraclavicular, or internal mammary lymph nodes. PMRT was administered in some cases where the surgical margins were exposed, or lymph nodes were positive for metastasis, but criteria for administering radiotherapy varied among institutions.
Statistical analysis
All data analyses were conducted using Stata statistical software (Stata SE 13.1; Stata Corp LP, College Station, TX, USA). The patients were divided into two groups: the NAC group consisted of patients who received NAC, the control group of patients who did not. The associations between clinicopathological factors and patient groups were statistically compared using Fisher's exact test or the Mann–Whitney U test. Similarly, Fisher's exact test was used to analyze the associations between postoperative complications and patient groups. Additionally, the odds ratios (ORs) were estimated using logistic regression analyses to assess the effects of variables such as BMI, smoking status, NAC, type of mastectomy or reconstruction, and PMRT on postoperative wound complications. Local recurrence-free survival (LRFS) and regional recurrence-free survival (RRFS) was calculated from the time of surgery until the detection of recurrence or the final contact with the patient. Survival curves and the cumulative incidence of events were generated using the Kaplan–Meier method. The differences in Kaplan–Meier curves were assessed using the log-rank test. The hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox regression model to identify potential prognostic indicators. To mitigate selection bias in the administration of NAC, we performed propensity score matching analysis with a logit-based model using the psmatch2 STATA function. The covariates for matching were pT, pN, hormone receptor and HER2 status as factors significantly different between the NAC and control groups. All statistical tests were two-sided, and a P value of less than 0.05 was considered to indicate a statistically significant association.
Results
In total 4,726 cases who underwent IBR were included in this study. Among them, 473 cases (10.0%) received NAC. The median age of patients in the NAC group was 45 years (range 24–77), while that of the control group was 46 years (range 20–83), (P < 0.001). Table 1 lists the characteristics of patients, tumors, and treatments for each group. There were significantly more cases with bilateral breast cancer in the control group (695 cases; 16.3%) than in the NAC group (60 cases; 12.7%) (P = 0.04). There were no significant differences between the two groups in terms of menopausal status, smoking habit, and BMI. NSM was performed significantly more frequently in the NAC group (190 cases; 40.2%), than in the control group (1241 cases; 29.2%) (P < 0.001). Reconstruction with autologous (including free-flap or pedicle-flap) or direct silicone breast implant (SBI) was performed significantly more frequently in the NAC group (96 cases; 20.3%, 51 case; 10.8%, respectively) than in the control group (427 cases; 10.0%, 115 cases; 2.7%, respectively) (P < 0.001). Reconstruction with tissue expander (TE) followed by SBI was performed significantly more frequently in the control group (3711 cases; 87.3%) than in the NAC group (326 cases; 68.9%) (P < 0.001). Axillary dissection was also performed significantly more frequently in the NAC group (260 cases; 55.0%) than in the control group (555 cases; 13.1%) (P < 0.001). Additionally, PMRT was performed significantly more frequently in the NAC group (115 cases; 24.3%) than in the control group (244 cases; 5.7%) (P < 0.001). The NAC group had significantly larger pathological tumor sizes than the control group (P < 0.001). Furthermore, the NAC group had higher proportions of tumors that were ER-negative (26.8%), PgR-negative (46.0%), HER2-positive (29.2%), and/or showed lymphovascular infiltration (30.0%), as compared to the control group (ER-negative, 11.4%; PgR-negative, 17.8%; HER2-positive, 12.6%; lymphovascular infiltration-positive, 22.5%). There was no significant difference in the frequency of tumor cells exposure at the surgical margin between the two groups, with 4.7% of the NAC group and 5.9% of the control group having positive surgical margins.
Safety
During a median follow-up period of 76.5 months (range 0–168), overall, postoperative complications were observed in 840 cases (17.8%). There were no significant differences in the incidence of complications between the NAC group (96 cases; 20.3%), and the control group (744 cases; 17.5%). We also assessed the correlations between postoperative complications and NAC regimens. Our results showed no significant correlations between them. Table 2 shows the associations between NAC and postoperative complications. The NAC group had significantly higher incidences of capsular contracture of the tissue expander (1.1%) and rupture (1.1%) of the SBI or TE than the control group, in which the incidences were 0.1% and 0.05%, respectively (P < 0.001, 0.001). In the NAC group, two of the four patients with capsular contracture had undergone PMRT, while the four with rupture had not. Table 3 shows the results of the regression analysis for postoperative complications, adjusted for factors such as BMI, smoking status, breast surgery type, reconstruction type, axillary surgery, NAC, PMRT and adjuvant chemotherapy. Overweight patients (OR 1.29; 95%CI 1.13–1.48) compared to those with normal BMI, those who underwent NSM/SSM (OR 1.60; 95%CI 1.45–1.76) compared to those who underwent total mastectomy, and those who received reconstruction with prosthesis (OR 1.28; 95%CI 1.15–1.43) compared to those who received reconstruction with autologous tissue, were found to have a significantly increased risk of developing postoperative complications. NAC was not, however, associated with an increased risk of postoperative complications.
Oncologic outcome
During a median follow-up period of 76.5 months (range 0–168), 36 patients (7.6%) in the NAC group and 147 (3.5%) in the control group developed local recurrence (P < 0.001). The 5-year LRFS was 93.1% (95% CI 0.90–0.95) in the NAC group and 97.1% (95% CI 0.97–0.98) in the control group. The rates of main local recurrence sites are as follows; subcutaneously fatty tissue, skin, nipple areola; 47.2%, 27.8%; 22.2% in the NAC group. Regional recurrence was detected in 35 patients (7.4%) in the NAC group and 85 (2.0%) in the control group (P < 0.001). The 5-year RRFS were 96.4% (95% CI 0.94–0.98) and 99.5% (95% CI 0.99–1.00). Kaplan–Meier survival curves for local recurrence (log-rank test, P < 0.001) and regional recurrence (log-rank test, P < 0.001) are shown in Fig. 1. Table 4 shows the results of univariate and multivariate analyses for LRFS and RRFS. Multivariate analysis for LRFS demonstrated pathological tumor size (HR 1.34; 95% CI 1.15–1.56), lymph node status (HR 0.70; 95% CI 0.52–0.95), lymphovascular invasion (HR 1.67; 95% CI 1.16–2.40), surgical technique (HR 1.81; 95% CI 1.49–2.22), PMRT (HR 0.18; 95% CI 0.07–0.44), and NAC (HR 2.00; 95% CI 1.33–3.04) to be factors significantly associated with local recurrence. After propensity score matching, however, there was no difference in local recurrence between the two groups. Multivariate analysis for RRFS demonstrated pathological tumor size (HR 1.31; 95% CI 1.08–1.59), lymphovascular invasion (HR 2.77; 95% CI 1.79–4.30), PMRT (HR 0.28; 95% CI 0.13–0.58), and NAC (HR 2.63; 95% CI 1.69–4.09) to be factors significantly associated with regional recurrence.
Discussion
This retrospective multicenter study showed that patients who received IBR after NAC did not have significantly higher rates of postoperative complications than those who did not receive NAC. To our knowledge, this is one of the largest studies to date evaluating the impact of NAC on the safety and long-term outcomes of patients undergoing IBR worldwide.
In this retrospective study, the postoperative complication rate was 20.3% in the NAC group. This result showed a favorable outcome compared to previous reports which indicated incidence of postoperative complications in patients who received NAC before IBR ranging from 23 to 39% [25,26,27,28]. In our study, the incidence of capsular contracture of the TE (1.1%) and ruptured prostheses (1.1%), including one TE and three SBI cases, were notably low in the NAC group; nonetheless, these rates were significantly higher than those in the control group. Capsular contracture of the SBI was not observed. However, it's important to note that the incidence of prosthesis loss (4.2%) and total flap loss (0%) was not significantly higher in the NAC group. These results also showed a favorable outcome compared to previous reports which indicated rates of prosthesis loss ranging from 8 to 26% and those of total flap loss ranging from 0 to 4% [26, 28]. We consider the following reasons for the rarity of capsular contracture of the SBI. In this study, we performed post-pectoral implant-based breast reconstruction using the textured surface implant, because the case collection period was before breast implant-associated anaplastic large cell lymphoma was reported, and the follow-up period (median; 76.5 months) may be inadequate.
Multivariate logistic regression analysis identified being over-weight, NSM/SSM, and reconstruction with TE/SBI as factors associated with an increased risk of complications. Our data and several meta-analyses focusing on IBR after NAC showed no significant increase in postoperative complications [21, 22].
The 5-year LRFS was 93.1% in the NAC group and 97.1% in the control group. The respective 5-year RRFS were 96.4% and 99.5%. The Cox regression model showed that the use of NAC was also a predictor of local and regional recurrences, along with large pathological tumor size, positive lymphovascular infiltration, NSM/SSM and no PMRT administration. The reasons for these observations could be attributed as follows; Firstly, patients who received NAC were more likely to have advanced stage breast cancer and to have hormone receptor negative and/or HER2 positive tumors with a high biological grade. However, following propensity score matching, the impact of NAC on local recurrence did not attain statistical significance. Secondary, patients receiving NAC more likely to undergo NSM, and in the NAC group, eight patients (4.2%) developed nipple recurrence, whereas this occurred in 11 patients (0.8%) in the control group. Thirdly, PMRT might be omitted in some cases for whom it was indicated due to fear of complications. To improve local control, PMRT was used for breast cancer patients with more than 4 positive nodes. The Japan Breast Cancer Society and National Comprehensive Cancer Network guidelines recommend using PMRT, regardless of the reconstruction approach [29, 30]. However, both patients and physicians might elect to forego PMRT if IBR has been undergone due to concerns regarding complications. In the American College of Surgeons Oncology Group Z1071 study, a prospective trial that evaluated the false negative rate of sentinel lymph node surgery after NAC among breast cancer patients with initial node positive disease, PMRT was significantly less common in patients undergoing IBR [31]. We need to provide meticulous follow-up for the patients who received NAC before IBR, particularly after undergoing NSM. However, several meta-analyses and recent reports have demonstrated that IBR after NAC had no impact on either overall or disease-free survival nor local recurrence [23,24,25]. Recently, Wu et al. examined oncological safety by applying the propensity score matching method to compare 323 patients who underwent IBR and 323 patients receiving conventional mastectomy alone after NAC. The median follow-up period in their study was 67 months. They experienced 3.7% of local recurrence, and 7.1% of regional recurrence in patients with IBR after NAC and found that there were no significant differences between the two groups in either local or regional recurrence, nor in rates of distant metastasis and overall survival [14]. In the past, a large proportion of patients receiving NAC undergo mastectomy as the surgical treatment either because breast- conserving surgery is not feasible or according to patient preference [32]. Although careful follow-up is required for post-operative local recurrence and adverse effects on TE and SBI, IBR now needs to be established as a standard procedure for patients treated with NAC to restore the physical image and positively influence the psychological well-being and quality of life of the patient.
The present study has several limitations. This was a retrospective study conducted at several institutions. Therapeutic strategies might have differed among them. However, the strengths of this study lie in the large number of subjects, the long period of follow-up and the detailed examination.
Conclusion
The findings of this large retrospective study suggest that IBR after NAC is a safe procedure with an acceptable postoperative complication profile. IBR has become now well established as one of the standard procedures for patients treated with NAC, although careful follow-up is required regarding postoperative locoregional recurrence and adverse effects on TE and SBI.
References
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15–23.
National Cancer Center Japan, Center for Cancer Control and Information Services https://ganjoho.jp/reg_stat/statistics/stat/summary.html
Bonadonna G, Valagussa P, Brambilla C, Ferrari LG. Preoperative chemotherapy in operable breast cancer. Lancet. 1993;341:1485.
Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32:1216–35.
Agostinetto E, Gligorov J, Piccart M. Systemic therapy for early-stage breast cancer: learning from the past to build the future Nat Rev. Clin Oncol. 2022;19:763–74.
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab Emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–28.
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–59.
Geyer CE Jr, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol. 2022;33:1250–68.
Johnston SRD, Toi M, O’Shaughnessy J, Rastogi P, Campone M, Neven P, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24:77–90.
Toi M, Imoto S, Ishida T, Ito Y, Iwata H, Masuda N, et al. Adjuvant S-1 plus endocrine therapy for oestrogen receptor-positive, HER2-negative, primary breast cancer: a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:74–84.
Al-Ghazal SK, Sully L, Fallowfield L, Blamey RW. The psychological impact of immediate rather than delayed breast reconstruction. Eur J Surg Oncol. 2000;26:17.
Atisha D, Alderman AK, Lowery JC, Kuhn LE, Davis J, Wikins EG. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year postoperative results from the Michigan breast reconstruction outcomes study. Ann Surg. 2008;247:1019–28.
Retrouvey H, Kerrebijn I, Metcalfe KA, O’Neill AC, McCready DR, Hofer SOP, et al. Psychosocial functioning in women with early breast cancer treated with breast surgery with or without immediate breast reconstruction. Ann Sur Oncol. 2019;26:2444–51.
Wu ZY, Kim HJ, Lee JW, Chung IY, Kim JS, Lee SB, et al. Long-term oncologic outcomes of immediate breast reconstruction vs conventional mastectomy alone for breast cancer in the setting of neoadjuvant chemotherapy. JAMA Surg. 2020;155:1142–50.
Retrouvey H, Solaja O, Gagliardi AR, Webster F, Zhong T. Barriers of access to breast reconstruction: a systematic review. Plast Reconstr Surg. 2019;143:465e–76e.
Yamakado R, Ishitobi M, Kondo N, Yamauchi C, Sasada S, Nogi H, et al. Physicians’ perception about the impact of breast reconstruction on patient prognosis: a survey in Japan. Breast Cancer. 2023;30:302–8.
Mitchem J, Herrmann D, Margenthaler JA, Aft RL. Impact of neoadjuvant chemotherapy on rate of tissue expander/implant loss and progression to successful breast reconstruction following mastectomy. Am J Surg. 2008;196:519–22.
Dolen UC, Schmidt AC, Um GT, Sharma K, Naugton M, Zoberi I, et al. Impact of neoadjuvant and adjuvant chemotherapy on immediate tissue expander breast reconstruction. Ann Surg Oncol. 2016;23:2357–66.
Eriksson M, Anveden L, Celebioglu F, Dahlberg K, Meldahl I, Lagergren J, et al. Radiotherapy in implant-based immediate breast reconstruction: risk factors, surgical outcomes, and patient-reported outcome measures in a large Swedish multicenter cohort. Breast Cancer Res Treat. 2013;142:591–601.
Cordeiro PG, Albornoz CR, McCormick B, Hu Q, Van Zee K. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg. 2014;134:588–95.
Varghese J, Gohari SS, Rizki H, et al. A systematic review and meta-analysis on the effect of neoadjuvant chemotherapy on complications following immediate breast reconstruction. Breast. 2021;55:55–62.
Lorentzen T, Heidemann LN, Möller S, Bille C. Impact of neoadjuvant chemotherapy on surgical complications in breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2022;48:44–52.
Donker M, Hage JJ, Woerdeman LA, Rutgers EJ, Sonke GS, Peeters MJ. Surgical complications of skin sparing mastectomy and immediate prosthetic reconstruction after neoadjuvant chemotherapy for invasive breast cancer. Eur J Surg Oncol. 2012;38:25–30.
Abt NB, Flores JM, Baltodano PA, Sarhane KA, Abreu FM, Cooney CM, et al. Neoadjuvant chemotherapy and short-term morbidity in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg. 2014;149:1068–76.
Teotia SS, Venutolo C, Haddock NT. Outcomes in patients receiving neoadjuvant chemotherapy undergoing immediate breast reconstruction: effect of timing, postoperative complications, and delay to radiation therapy. Plast Reconstr Surg. 2019;144:732e-e742.
Riba J, de Romani SE, Masia J. Neoadjuvant chemotherapy for breast cancer treatment and the evidence-based interaction with immediate autologous and implant-based breast reconstruction. Clin Plast Surg. 2018;45:25–31.
Ryu JM, Park S, Paik HJ, Nam SJ, Kim SW, Lee SK, et al. Oncologic safety of immediate breast reconstruction in breast cancer patients who underwent neoadjuvant chemotherapy: short-term outcomes of a matched case-control study. Clin Breast Cancer. 2017;17:204–10.
Wengler CA, Valente SA, Al-Hilli Z, Woody NM, Muntean JH, Abraham J, et al. Determinants of short and long term outcomes in patients undergoing immediate breast reconstruction following neoadjuvant chemotherapy. J Surg Oncol. 2017;116:797–802.
Inokuchi M, Kutomi G, Kijima Y, Sakai T, Sawaki M, Shien T, et al. The Japanese Breast Cancer Society clinical practice guidelines for surgical treatment of breast cancer, 2018 edition. Breast Cancer. 2020;27:4–8.
National Comprehensive Cancer Network: NCCN guidelines. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 1 June 2023.
Haffty BG, McCall LM, Ballman KV, McLaughlin S, Jagsi R, Ollila DW, et al. Patterns of local-regional management following neoadjuvant chemotherapy in breast cancer: results from ACOSOG Z1071 (Alliance). Int J Radiat Oncol Biol Phys. 2016;94:493–502.
Hoffman KE, Mittendorf EA, Buchholz TA. Optimising radiation treatment decisions for patients who receive neoadjuvant chemotherapy and mastectomy. Lancet Oncol. 2012;13:e270–6.
Acknowledgements
We thank to the scientific committee of the Japanese Breast Cancer Society.
Funding
This study was supported by a grant from the scientific committee of the Japanese Breast Cancer Society.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Nogi, H., Ogiya, A., Ishitobi, M. et al. Impact of neoadjuvant chemotherapy on the safety and long-term outcomes of patients undergoing immediate breast reconstruction after mastectomy. Breast Cancer 31, 507–518 (2024). https://doi.org/10.1007/s12282-024-01570-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-024-01570-w