Abstract
Background
Mean platelet volume (MPV) is one of the four platelet parameters (platelet count, MPV, platelet distribution width and plateletcrit), which indicates the activation of platelet. We aim to investigate the associations between pre-treatment MPV levels and clinical hematology parameters, pathology parameters and prognosis of patients with invasive breast cancer (IBC).
Methods
Medical records of 340 breast tumor patients (170 IBC vs. 170 breast benign tumor) were retrospectively reviewed. Patients in two groups were matched for age, body mass index, smoking status and complications. To analyze: differences in pre-treatment MPV levels between IBC group and breast benign tumor group; differences between pre- and postoperative MPV levels in IBC patients; correlations between pre-treatment MPV and clinical hematology parameters, clinicopathologic parameters and prognosis in IBC patients.
Results
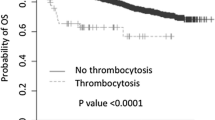
As we analyzed, pre-treatment MPV levels of IBC patients were significantly higher than the controls (8.65 ± 0.98 vs 8.34 ± 0.78, P = 0.002), and preoperative MPV levels were significantly higher than the postoperative in IBC patients (8.65 ± 0.98 vs 8.44 ± 0.91, P = 0.042). In IBC group, pre-treatment MPV level associated, significantly, with clinical hematology parameters (platelet, fibrinogen, albumin, fasting blood glucose, P = 0.003, 0.042, 0.032, 0.046, respectively) and with clinicopathological parameters (distant metastasis, primary tumor size, tumor node metastasis stages, P = 0.039, 0.002, 0.001, respectively). Furthermore, univariate and multivariate survival analysis demonstrated that MPV was significant prognostic factor (P = 0.035, HR 1.86, 95 % confidence interval 1.06–3.25).
Conclusion
High pre-treatment MPV level in IBC patients was a potential predictive factor and significant independent prognostic factor.


Similar content being viewed by others
References
Tran BH, Joanna Nguyen T, Hwang BH, Vidar EN, Davis GB, Chan LS, et al. Risk factors associated with venous thromboembolism in 49,028 mastectomy patients. Breast. 2013;22(4):444–8.
Andtbacka RH, Babiera G, Singletary SE, Hunt KK, Meric-Bernstam F, Feig BW, et al. Incidence and prevention of venous thromboembolism in patients undergoing breast cancer surgery and treated according to clinical pathways. Ann Surg. 2006;243(1):96–101.
Clahsen PC, van de Velde CJ, Julien JP, Floiras JL, Mignolet FY. Thromboembolic complications after perioperative chemotherapy in women with early breast cancer: a European organization for research and treatment of cancer breast cancer cooperative group study. J Clin Oncol. 1994;12(6):1266–71.
Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320(8):479–84.
Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339–46.
Wedgwood KR, Benson EA. Non-tumour morbidity and mortality after modified radical mastectomy. Ann R Coll Surg Engl. 1992;74(5):314–7.
Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012–9.
Biggerstaff JP, Seth N, Amirkhosravi A, Amaya M, Fogarty S, Meyer TV, et al. Soluble fibrin augments platelet/tumor cell adherence in vitro and in vivo, and enhances experimental metastasis. Clin Exp Metastasis. 1999;17:723–30.
Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98:3352–7.
Falanga A, Panova-Noeva M, Russo L. Procoagulant mechanisms in tumour cells. Best Pract Res Clin Haematol. 2009;22(1):49–60.
Jakubowski JA, Thompson CB, Vaillancourt R, Valeri CR, Deykin D. Arachidonic acid metabolism by platelets of differing size. Br J Haematol. 1983;53:503–11.
Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338:1409–11.
Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–56.
Greisenegger S, Endler G, Hsieh K. Is high mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35:1688–91.
Kapsoritakis AN, Koukourakis MI, Sfiridaki Potamianos SP, Kosmadaki MG, Koutroubakis IEA, et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96(3):776–81.
Kisacik Bunyamin, Tufan Abdurrahman, Kalyoncu Umut, Karadag O, Akdogan A, Ozturk MA, et al. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Jt Bone Spine. 2008;75:291–4.
Kurt M, Onal IK, Sayilir AY, Beyazit Y, Oztas E, Kekilli M, et al. The role of mean platelet volume in the diagnosis of hepatocellular carcinoma in patients with chronic liver disease. Hepatogastroenterology. 2012;59(117):1580–2.
Jia-Ying Li, Ying Li, Zheng Jiang, Rui-Tao Wang, Xi-Shan Wang, et al. High mean platelet volume is associated with presence of colon cancer. Asian Pac J Cancer Prev. 2014;15(23):10501–4.
Matowicka-Karna Joanna, Kamocki Zbigniew, PoliNska Beata, Osada J, Kemona H. Platelets and inflammatory markers in patients with gastric cancer. Clin Dev Immunol. 2013;2013:401623–8.
Inagaki N, Kibata K, Tamaki T, Shimizu T, Nomura S. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer. 2014;83(1):97–101.
Karaman K, Bostanci EB, Aksoy E, Kurt M, Celep B, Ulas M, et al. The predictive value of mean platelet volume in differential diagnosis of non-functional pancreatic neuroendocrine tumors from pancreatic adenocarcinomas. Eur J Intern Med. 2011;22(6):e95–8.
Vizioli L, Muscari S, Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. 2009;63(10):1509–15.
Lancé MD, van Oerle R, Henskens YM, Marcus MA. Do we need time adjusted mean platelet volume measurements? Lab Hematol. 2010;16(3):28–31.
Berger FG. The interleukin-6 gene: a susceptibility factor that may contribute to racial and ethnic disparities in breast cancer mortality. Breast Cancer Res Treat. 2004;88:281–5.
Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:V215–24.
Riedl J, Kaider A, Reitter EM, Marosi C, Jäger U, Schwarzinger I, et al. Association of mean platelet volume with risk of venous thromboembolism and mortality in patients with cancer. Results from the Vienna Cancer and Thrombosis Study (CATS). Thromb Haemost. 2014;111(4):670–8.
Mutlu H, Artis TA, Erden A, Akca Z. Alteration in mean platelet volume and platicrit values in patients with cancer that developed thrombosis. Clin Appl Thromb Hemost. 2013;19(3):331–3.
Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin (ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–85.
Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69.
Hekimsoy Z, Payzin B, Ornek T, Kandofan G. Mean platelet volume in type 2 diabetic patients. J Diabetes Complicat. 2004;18(3):173–6.
Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T, et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475–8.
Kuderer NM, Khorana AA, Lyman GH, Francis CW, et al. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: impact on survival and bleeding complications. Cancer. 2007;110:1149–61.
Lazo-Langner A, Goss GD, Spaans JN, Hodsman A, Kovacs MJ, Clement AM, et al. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost. 2007;5:729–37.
Falanga A, Piccioli A. Effect of anticoagulant drugs in cancer. Curr Opin Pulm Med. 2005;11:403–7.
Acknowledgments
This study was supported by Natural Science Foundation Project of Liaoning Province (No. 2010010280-401).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
About this article
Cite this article
Gu, M., Zhai, Z., Huang, L. et al. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer 23, 752–760 (2016). https://doi.org/10.1007/s12282-015-0635-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-015-0635-6