Abstract
Background
HER2 expression is an important prognostic and predictive factor of treatment efficacy in breast cancer. Trastuzumab, in particular, is a key drug in the treatment of HER2-positive recurrent breast cancer. However, the difference in treatment efficacy between trastuzumab monotherapy and combination therapy with chemotherapy is unclear. In order to elucidate this point, both treatments were compared in terms of efficacy by metastatic site, time to progression (TTP), and survival.
Patients and methods
The subjects were 1,471 breast cancer patients who had been evaluated for HER2 expression between 1998 and March 2006; 74 of these had recurrent breast cancer that had been treated with trastuzumab. Of these 74 patients, 39 received trastuzumab alone and 45 trastuzumab in combination with chemotherapy. The items of investigation were clinical effect, TTP, survival, biological markers such as ER/PgR, proliferation (Ki67) or p53 overexpression, nuclear grade, performance status (PS), lymph node metastasis, and tumor size.
Results
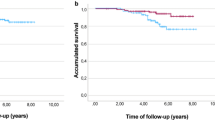
The HER2-positive rate was 23.3%, and the degree of malignancy in these HER2-positive patients was high; postoperative disease-free survival (DFS) was low. However, this tendency was clear in patients with hormone-responsive breast cancer. In patients with hormone-non-responsive breast cancer, HER2 negativity had a significantly higher Ki67 value, and there was no difference in DFS between patients with HER2-positive and -negative tumors. Among the 74 patients with recurrent breast cancer, the response rate to trastuzumab was 64.9%; however, among patients who received the combination treatment, the response rate was 86%. In patients with liver metastasis, the effect of trastuzumab alone was low, but that of the combination treatment was significantly high. TTP was 5.7 months and 15.9 months with trastuzumab alone and the combination therapy, respectively. Furthermore, a significant difference was seen in post-treatment survival; however, there was no significant difference in survival after a recurrence. In the multivariate analysis on factors for TTP, PS, clinical effect, and combination treatment were significant. However, good PS and early treatment were the significant factors in post-treatment survival.
Conclusions
The effect of trastuzumab in patients with recurrent breast cancer who received the combination treatment was significantly high and TTP was long. However, this was not a significant factor in terms of overall survival. In particular, a good PS and early treatment were important in post-treatment survival.


Similar content being viewed by others
References
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235(4785):177–82.
Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244(4905):707–12.
Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol 2001;12(suppl 1):S3–S8.
Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta 1994;1198(2–3):165–84.
Pauletti G, Godolphin W, Press MF, et al. Detection and quantization of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene 1996;13(1):63–72.
Hynes NE, Gerber HA, Saurer S, et al. Overexpression of the c-erbB-2 protein in human breast tumor cell lines. J Cell Biochem 1989;39(2):167–73.
Hynes NE. Amplification and overexpression of the erbB-2 gene in human tumors: its involvement in tumor development, significance as a prognostic factor, and potential as a target for cancer therapy. Semin Cancer Biol 1994;4(1):19–26.
Di Leo A, Larsimont D, Beauduin M, et al. CMF or anthracycline-based adjuvant chemotherapy for node-positive breast cancer patients: 4 year results of a Belgian randomized clinical trial with predictive markers analysis. Proc Am Soc Clin Oncol 1999;18:69a (abstract 258).
Gusterson BA, Gelber RD, Goldhirsch A, et al. Prognostic importance of c-erbB-2 expression in breast cancer: International (Ludwig) Breast Cancer Study Group. J Clin Oncol 1992;10:1049–56.
Ravdin PM. Prognostic factors in breast cancer In: Perry MC (eds) 1997 ASCO Educational Book. Alexandria: American Society of Clinical Oncology; 1997, pp. 217–27.
Elledge RM, Green S, Ciocca D, et al. HER-2 expression and response to tamoxifen in estrogen receptor-positive breast cancer: a southwest oncology group study. Clin Cancer Res 1998;4:7–12.
Dieras V, de Cremoux D, Le Doussal V, et al. Is erbB-2 a predictive marker for response to primary chemotherapy (CT) for operable breast cancer? Prospective study in a phase III randomized, parallel study of doxorubicin/cyclophosphamide (AC) and doxorubicin/Taxol® (paclitaxel) (AT). Proc Am Soc Clin Oncol 1999;18:85a (abstract 322).
Paik S, Bryant J, Park C, et al. erbB-2 and response to doxorubicin in patients with axillary lymph node-positive, hormone receptor-negative breast cancer. J Natl Cancer Inst 1998;90(18):1361–70.
Petit T, Ghnassia JP, Rodier JF, et al. Relationship between erbB-2 status and neoadjuvant chemotherapy response is dependent on anthracycline dose intensity. Proc Am Soc Clin Oncol 2000;19:96a (abstract 370).
Ravdin PM, Green S, Albain KS, et al. Initial report of the SWOG biological correlative study of c-erbB-2 expression as a predictor of outcome in a trial comparing adjuvant CAF T with tamoxifen (T) alone. Proc Am Soc Clin Oncol 1998;17:97a (abstract 374).
Untch M, Thomssen C, Kahlert D, et al. Lack of c-erbB-2 overexpression predicts better response to dose intensification of anthracycline-based chemotherapy in high-risk breast cancer. Breast Cancer Res Treat 1998;50:238 (abstract 110).
Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 2004;96(10):739–49.
Pegram M, Hsu S, Lewis G, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 1999;18(13):2241–51.
Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002;20:719–26.
Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999;17:2639–48.
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92.
Marty M, Cognetti F, Maraninchi D, et al. Randomized Phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005;232:4265–74.
Pegram M. Docetaxel and herceptin: foundation for future strategies. Oncologist 2001;6:22–5.
Baselga J. Herceptin alone or in combination with chemotherapy in the treatment of HER2-positive metastatic breast cancer: pivotal trials. Oncology 2001;61(Suppl 2):14–21.
Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl Med 2005;353:1673–84.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659–72.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is based on a presentation delivered at Symposium 3, “Molecular target therapy: basics and clinical application,” held on 30 June 2007 at the 15th Annual Meeting of the Japanese Breast Cancer Society in Yokohama.
About this article
Cite this article
Nishimura, R., Okumura, Y. & Arima, N. Trastuzumab monotherapy versus combination therapy for treating recurrent breast cancer: time to progression and survival. Breast Cancer 15, 57–64 (2008). https://doi.org/10.1007/s12282-007-0014-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-007-0014-z