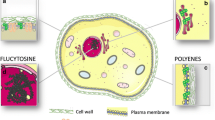
Abstract
Advances in medicine have led to more patients being at risk of fungal infections. Diagnostic tools are limited, but early antifungal treatment is crucial to improve outcome. Hence, not a few patients receive empirical antifungals with the disadvantage of increasing the burden of antifungal drug resistance. From a clinical point of view, it is of interest to understand how commonly resistance occurs, how easy it is induced through therapy, and how often it results in clinical treatment failure. The answer differs and depends on the clinical setting, the type of fungal disease, the class of antifungal agent, and treatment duration. This review provides a comprehensive overview on cross-resistance (CR) and multidrug resistance (MR) occurring in Candida species. Known amino acid substitutions are listed which lead to CR (resistance against ≥two azoles or echinocandins), pan-azole resistance (against all systemically applied azoles), pan-echinocandin resistance (against all echinocandins), or MR (polyene-azole resistance, 5-fluorouracil-azole resistance, and azole-echinocandin resistance). Data are supplemented with treatment results from animal studies and experiences from various case reports. An appraisal will be made based on the current frequency of CR and MR reported in the literature, and subsequently, the impact of CR and MR on patient management will be discussed.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Arendrup MC. Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect. 2014;20 Suppl 6:42–8. doi:10.1111/1469-0691.12513. This article is of special interest as it provides a comprehensive overview on modified clinical breakpoints for Aspergillus and Candida.
Glockner A. Treatment and prophylaxis of invasive candidiasis with anidulafungin, caspofungin and micafungin: review of the literature. Eur J Med Res. 2011;16(4):167–79.
Schmalreck AF, Lackner M, Becker K, et al. Phylogenetic relationships matter: antifungal susceptibility among clinically relevant yeasts. Antimicrob Agents Chemother. 2014;58(3):1575–85. This article summarizes all currently accepted changes in nomenclature among clinically relevant ascomycetous yeasts. Moreover, a correlation between the evolutionary relatedness of yeast and their antifungal susceptibility profiles.
Pfaller MA, Diekema DJ, Andes D, et al. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat. 2011;14(3):164–76.
Pfaller MA, Andes D, Arendrup MC, et al. Clinical breakpoints for voriconazole and Candida spp. revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn Microbiol Infect Dis. 2011;70(3):330–43.
White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402.
Arendrup MC, Garcia-Effron G, Buzina W, et al. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob Agents Chemother. 2009;53(3):1185–93.
Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med. 2012;125:S3–13.
Maubon D, Garnaud C, Calandra T, et al. Resistance of Candida spp. to antifungal drugs in the ICU: where are we now? Intensiv Care Med. 2014;40(9):1241–55.
Pfaller MA, Castanheira M, Lockhart SR, et al. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol. 2012;50(4):1199–203.
Rex JH, Pfaller MA, Galgiani JN, et al. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin Infect Dis. 1997;24(2):235–47.
de Hoog GS et al. Name changes in medically important fungi and their implication on clinical practice. J Clin Microbiol. 2014; accepted for publication.
Lortholary O, Desnos-Ollivier M, Sitbon K, et al. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob Agents Chemother. 2011;55:532–8.
Blanchard E, Lortholary O, Boukris-Sitbon K, et al. Prior caspofungin exposure in patients with hematological malignancies is a risk factor for subsequent fungemia due to decreased susceptibility in Candida spp.: a case-control study in Paris, France. Antimicrob Agents Chemother. 2011;55:5358–61.
Pfeiffer CD, Garcia-Effron G, Zaas AK, et al. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol. 2010;48(7):2373–80.
Slater JL, Howard SJ, Sharp A, et al. Disseminated Candidiasis caused by Candida albicans with amino acid substitutions in FKS1 at position Ser645 cannot be successfully treated with micafungin. Antimicrob Agents Chemother. 2011;55(7):3075–83.
Spreghini E, Orlando F, Sanguinetti M, et al. Comparative effects of micafungin, caspofungin, and anidulafungin against a difficult-to-treat fungal opportunistic pathogen, Candida glabrata. Antimicrob Agents Chemother. 2012;56(3):1215–22.
Tripathi N, Watt K, Benjamin Jr DK. Treatment and prophylaxis of invasive candidiasis. Semin Perinatol. 2012;36(6):416–23.
Ruggero MA, Topal JE. Development of echinocandin-resistant Candida albicans candidemia following brief prophylactic exposure to micafungin therapy. Transpl Infect Dis. 2014;16(3):469–72.
Beyda ND, Lewis RE, Garey KW. Echinocandin resistance in Candida species: mechanisms of reduced susceptibility and therapeutic approaches. Ann Pharmacother. 2012;46(7–8):1086–96.
Fekkar A, Dannaoui E, Meyer I, et al. Emergence of echinocandin-resistant Candida spp. in a hospital setting: a consequence of 10 years of increasing use of antifungal therapy? Eur J Clin Microbiol Infect Dis. 2014;33(9):1489–96. The article provides real-life clinical experience on fungal prophylaxis and its association with the development of antifungal resistances among Candida species.
Cavling Arendrup M, Cuenca-Estrella M, Lass-Florl C, Hope WW. European Committee on Antimicrobial Susceptibility Testing—Subcommittee on Antifungal Susceptibility T. EUCAST technical note on Candida and micafungin, anidulafungin and fluconazole. Mycoses. 2014;57(6):377–9.
Bizerra FC, Jimenez-Ortigosa C, Souza ACR, et al. Breakthrough candidemia due to multidrug resistant C. glabrata during prophylaxis with low dose of micafungin. Antimicrob Agents Chemother. 2014;58:2438–40.
Alexander BD, Schell WA, Miller JL, et al. Candida glabrata fungemia in transplant patients receiving voriconazole after fluconazole. Transplantation. 2005;80:868–71.
White TC, Holleman S, Dy F, et al. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46(6):1704–13.
Sheikh N, Jahagirdar V, Kothadia S, Nagoba B. Antifungal drug resistance in Candida species. Eur J Gen Med. 2013;10(4):254–8.
Lackner M, Tscherner M, Schaller M, et al. Positions and numbers of FKS mutations in Candida albicans selectively influence in vitro and in vivo susceptibilities to echinocandin treatment. Antimicrob Agents Chemother. 2014;58(7):3626–35. This article shows the difference between heterozygote and homozygote mutations and their impact on in vitro and in vivo resistance. Moreover, it demonstrates that resistance is acquired during long-term therapy in chronically infected patients.
Pfaller MA, Messer SA, Moet GJ, et al. Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009). Int J Antimicrob Agents. 2011;38(1):65–9.
Pfaller MA, Diekema DJ. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol. 2012;50(9):2846–56.
Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin Microbiol Infect. 2008;14(4):398–405.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. 3rd ed. CLSI document M27-A3. Wayne: Clinical and Laboratory Standards Institute; 2008
van Hal SJ, Chen SC, Sorrell TC, et al. Support for the EUCAST and revised CLSI fluconazole clinical breakpoints by Sensititre® YeastOne® for Candida albicans: a prospective observational cohort study. J Antimicrob Chemother. 2014;69(8):2210–4.
Eschenauer GA, Nguyen MH, Shoham S, et al. Real-world experience with echinocandin MICs against Candida species in a multicenter study of hospitals that routinely perform susceptibility testing of bloodstream isolates. Antimicrob Agents Chemother. 2014;58(4):1897–906.
Fothergill AW, Sutton DA, McCarthy DI, Wiederhold NP. Impact of new antifungal breakpoints on antifungal resistance in Candida species. J Clin Microbiol. 2014;52(3):994–7.
Chen TC, Chen YH, Chen YC, Lu PL. Fluconazole exposure rather than clonal spreading is correlated with the emergence of Candida glabrata with cross-resistance to triazole antifungal agents. Kaohsiung J Med Sci. 2012;28(6):306–15.
Pham CD, Iqbal N, Bolden CB, et al. Role of FKS Mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother. 2014;58(8):4690–6.
Couzigou C, Gabriel F, Biteau N, et al. Two missense mutations, E123Q and K151E, identified in the ERG11 allele of an azole-resistant isolate of Candida kefyr recovered from a stem cell transplant patient for acute myeloid leukemia. Med Mycol Case Rep. 2014;5:12–5.
Ricardo E, Miranda IM, Faria-Ramos I, et al. In vivo and in vitro acquisition of resistance to voriconazole by Candida krusei. Antimicrob Agents Chemother. 2014;58(8):4604–11.
Strzelczyk JK, Slemp-Migiel A, Rother M, et al. Nucleotide substitutions in the Candida albicans ERG11 gene of azole-susceptible and azole-resistant clinical isolates. Acta Biochim Pol. 2013;60(4):547–52.
Chong Y, Shimoda S, Yakushiji H, et al. Fatal candidemia caused by azole-resistant Candida tropicalis in patients with hematological malignancies. J Infect Chemother. 2012;18(5):741–6.
Fanci R. Breakthrough Candida dubliniensis fungemia in an acute myeloid leukemia patient during voriconazole therapy successfully treated with caspofungin. J Chemother. 2009;21(1):105–7.
Krcmery V, Demitrovicova A, Kisac P. Breakthrough fungemia due to Candida glabrata during posaconazole prophylaxis in hematology patients treated with anidulafungin—report of 5 cases. J Chemother. 2011;23(5):310–1.
Myoken Y, Kyo T, Sugata T, et al. Breakthrough fungemia caused by fluconazole-resistant Candida albicans with decreased susceptibility to voriconazole in patients with hematologic malignancies. Haematologica. 2006;91(2):287–8.
Trifilio S, Singhal S, Williams S, et al. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transplant. 2007;40(5):451–6.
Linares CE, Giacomelli SR, Altenhofen D, et al. Fluconazole and amphotericin-B resistance are associated with increased catalase and superoxide dismutase activity in Candida albicans and Candida dubliniensis. Rev Soc Bras Med Trop. 2013;46(6):752–8.
Forastiero A, Mesa-Arango AC, Alastruey-Izquierdo A, et al. Candida tropicalis antifungal cross-resistance is related to different azole target (ERG11p) modifications. Antimicrob Agents Chemother. 2013;57(10):4769–81.
Hull CM, Parker JE, Bader O, et al. Facultative sterol uptake in an ergosterol-deficient clinical isolate of Candida glabrata harboring a missense mutation in ERG11 and exhibiting cross-resistance to azoles and amphotericin B. Antimicrob Agents Chemother. 2012;56(8):4223–32.
Sanglard D, Ischer F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40(10):2300–5.
Dogra S, Krishnamurthy S, Gupta V, et al. Asymmetric distribution of phosphatidylethanolamine in C. albicans: possible mediation by CDR1, a multidrug transporter belonging to ATP binding cassette (ABC) superfamily. Yeast. 1999;15(2):111–21.
Maesaki S, Marichal P, Vanden Bossche H, et al. Rhodamine 6G efflux for the detection of CDR1-overexpressing azole-resistant Candida albicans strains. J Antimicrob Chemother. 1999;44(1):27–31.
Prasad R, Singh A. Lipids of Candida albicans and their role in multidrug resistance. Curr Genet. 2013;59(4):243–50.
Dhamgaye S, Bernard M, Lelandais G, et al. RNA sequencing revealed novel actors of the acquisition of drug resistance in Candida albicans. BMC Genomics. 2012;13:396.
Wilke M. Treatment and prophylaxis of invasive candidiasis with anidulafungin, caspofungin and micafungin and its impact on use and costs: review of the literature. Eur J Med Res. 2011;16(4):180–6.
Bizerra FC, Jimenez-Ortigosa C, Souza AC, et al. Breakthrough candidemia due to multidrug-resistant Candida glabrata during prophylaxis with a low dose of micafungin. Antimicrob Agents Chemother. 2014;58(4):2438–40.
Chan TS, Gill H, Hwang YY, et al. Breakthrough invasive fungal diseases during echinocandin treatment in high-risk hospitalized hematologic patients. Ann Hematol. 2014;93(3):493–8.
Chrenkova V, Hubacek P, Sedlacek P, et al. Post-mortem analysis of Candida albicans breakthrough infection during echinocandin treatment in haematopoietic stem cell transplant recipient. Epidemiol Mikrobiol Imunol. 2014;63(2):121–4.
Fekkar A, Meyer I, Brossas JY, et al. Rapid emergence of echinocandin resistance during Candida kefyr fungemia treatment with caspofungin. Antimicrob Agents Chemother. 2013;57(5):2380–2.
Garcia-Effron G, Lee S, Park S, et al. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-D-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother. 2009;53(9):3690–9.
Park S, Kelly R, Kahn JN, et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother. 2005;49(8):3264–73.
Katiyar SK, Alastruey-Izquierdo A, Healey KR, et al. FKS1 and FKS2 are functionally redundant but differentially regulated in Candida glabrata: implications for echinocandin resistance. Antimicrob Agents Chemother. 2012;56(12):6304–9.
Staab JF, Neofytos D, Rhee P, et al. Target enzyme mutations confer differential echinocandin susceptibilities in Candida kefyr. Antimicrob Agents Chemother. 2014;58(9):5421–7.
Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS. A naturally occurring proline-to-alanine amino acid change in FKS1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Agents Chemother. 2008;52(7):2305–12.
Chapeland-Leclerc F, Hennequin C, Papon N, et al. Acquisition of flucytosine, azole, and caspofungin resistance in Candida glabrata bloodstream isolates serially obtained from a hematopoietic stem cell transplant recipient. Antimicrob Agents Chemother. 2010;54(3):1360–2.
Sun HY, Singh N. Characterisation of breakthrough invasive mycoses in echinocandin recipients: an evidence-based review. Int J Antimicrob Agents. 2010;35(3):211–8.
Martel CM, Parker JE, Bader O, et al. A clinical isolate of Candida albicans with mutations in ERG11 (encoding sterol 14alpha-demethylase) and ERG5 (encoding C22 desaturase) is cross resistant to azoles and amphotericin B. Antimicrob Agents Chemother. 2010;54(9):3578–83.
Eddouzi J, Parker JE, Vale-Silva LA, et al. Molecular mechanisms of drug resistance in clinical Candida species isolated from Tunisian hospitals. Antimicrob Agents Chemother. 2013;57(7):3182–93.
Gabriel F, Sabra A, El-Kirat-Chatel S, et al. Deletion of the uracil permease gene confers cross-resistance to 5-fluorouracil and azoles in Candida lusitaniae and highlights antagonistic interaction between fluorinated nucleotides and fluconazole. Antimicrob Agents Chemother. 2014;58(8):4476–85.
Pfeiffer CD, Garcia-Effron G, Zaas AK, et al. Breakthrough invasive candidiasis in patients on micafungin. J Clin Microbiol. 2010;48:2373–80.
Hakki M, Staab JF, Marr KA. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob Agents Chemother. 2006;50:2522–4.
Krogh-Madsen M, Arendrup MC, Heslet L, Knudsen JD. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin Infect Dis. 2006;42:938–44.
Cleary JD, Garcia-Effron G, Chapman SW, Perlin DS. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob Agents Chemother. 2008;52:2263–5.
Cleveland AA, Farley MM, Harrison LH, et al. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis. 2012;55:1352–61.
Cernicka J, Subik J. Resistance mechanisms in fluconazole-resistant Candida albicans isolates from vaginal candidiasis. Int J Antimicrob Agents. 2006;27(5):403–8.
Chau AS, Mendrick CA, Sabatelli FJ, et al. Application of real-time quantitative PCR to molecular analysis of Candida albicans strains exhibiting reduced susceptibility to azoles. Antimicrob Agents Chemother. 2004;48(6):2124–31.
Wang H, Kong F, Sorrell TC, et al. Rapid detection of ERG11 gene mutations in clinical Candida albicans isolates with reduced susceptibility to fluconazole by rolling circle amplification and DNA sequencing. BMC Microbiol. 2009;14(9):167.
Feng LJ, Wan Z, Wang XH, et al. Relationship between antifungal resistance of fluconazole resistant Candida albicans and mutations in ERG11 gene. Chin Med J (Engl). 2010;123(5):544–8.
Favre B, Didmon M, Ryder NS. Multiple amino acid substitutions in lanosterol 14alpha-demethylase contribute to azole resistance in Candida albicans. Microbiology. 1999;145(10):2715–25.
Löffler J, Kelly SL, Hebart H, et al. Molecular analysis of CYP51 from fluconazole-resistant Candida albicans strains. FEMS Microbiol Lett. 1997;151(2):263–8.
Xiang MJ, Liu JY, Ni PH, et al. ERG11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS Yeast Res. 2013;13(4):386–93.
Oliveira Carvalho V, Okay TS, Melhem MS, et al. The new mutation L321F in Candida albicans ERG11 gene may be associated with fluconazole resistance. Rev Iberoam Micol. 2013;30(3):209–12.
Morio F, Loge C, Besse B, et al. Screening for amino acid substitutions in the Candida albicans Erg11 protein of azole-susceptible and azole-resistant clinical isolates: new substitutions and a review of the literature. Diagn Microbiol Infect Dis. 2010;66(4):373–84.
Marichal P, Koymans L, Willemsens S, et al. Contribution of mutations in the cytochrome P450 14alpha-demethylase (ERG11p, CYP51p) to azole resistance in Candida albicans. Microbiology. 1999;145(Pt10):2701–13.
Manastir L, Ergon MC, Yücesoy M. Investigation of mutations in ERG11 gene of fluconazole resistant Candida albicans isolates from Turkish hospitals. Mycoses. 2011;54(2):99–104.
Goldman GH, Da Silva Ferreira ME, dos Reis Marques E, et al. Evaluation of fluconazole resistance mechanisms in Candida albicans clinical isolates from HIV-infected patients in Brazil. Diagn Microbiol Infect Dis. 2004;50(1):25–32.
Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42(2):241–53.
Ying Y, Zhao Y, Hu X, et al. In vitro fluconazole susceptibility of 1,903 clinical isolates of Candida albicans and the identification of ERG11 mutations. Microb Drug Resist. 2013;19(4):266–73.
Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW. Breakpoints for antifungal agents: an update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resist Updat. 2013;16(6):81–95.
Garcia-Effron G, Chua DJ, Tomada JR, et al. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob Agents Chemother. 2010;54(5):2225–7.
Jensen RH, Johansen HK, Arendrup MC. Stepwise development of a homozygous S80P substitution in FKS1p, conferring echinocandin resistance in Candida tropicalis. Antimicrob Agents Chemother. 2013;57(1):614–7.
Pfaller MA, Diekema DJ, Jones RN, Castanheira M. Use of anidulafungin as a surrogate marker to predict susceptibility and resistance to caspofungin among 4,290 clinical isolates of Candida using CLSI methods and interpretive criteria. J Clin Microbiol. 2014;52(9):3223–9.
Dannaoui E, Desnos-Ollivier M, Garcia-Hermoso D, et al. Candida spp. with acquired echinocandin resistance, France, 2004–2010. Emerg Infect Dis. 2012;18(1):86–90.
Desnos-Ollivier M, Bretagne S, Raoux D, et al. Mutations in the FKS1 gene in Candida albicans, C. tropicalis, and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the method of the European Committee on Antibiotic Susceptibility Testing. Antimicrob Agents Chemother. 2008;52(9):3092–8.
Zimbeck AJ, Iqbal N, Ahlquist AM, et al. FKS mutations and elevated echinocandin MIC values among Candida glabrata isolates from U.S. population-based surveillance. Antimicrob Agents Chemother. 2010;54(12):5042–7.
Fernandez-Silva F, Lackner M, Capilla J, et al. In vitro Antifungal susceptibility of Candida glabrata to caspofungin and the presence of FKS mutations correlate with treatment response in an immunocompromised murine model of invasive infection. Antimicrob Agents Chemother. 2014;58(7):3646–9.
Cleary JD, Garcia-Effron G, Chapman SW, Perlin DS. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob Agents Chemother. 2008;52(6):2263–5.
Castanheira M, Woosley LN, Diekema DJ, et al. Low prevalence of FKS1 hot spot 1 mutations in a worldwide collection of Candida strains. Antimicrob Agents Chemother. 2010;54(6):2655–9.
Shields RK, Nguyen MH, Press EG, et al. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob Agents Chemother. 2012;56(9):4862–9.
Garcia-Effron G, Kontoyiannis DP, Lewis RE, Perlin DS, et al. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob Agents Chemother. 2008;52(11):4181–3.
Garcia-Effron G, Park S, Perlin DS. Correlating echinocandin MIC and kinetic inhibition of FKS1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother. 2009;53(1):112–22.
Laverdiere M, Lalonde RG, Baril JG, et al. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J Antimicrob Chemother. 2006;57(4):705–8.
Prigitano A, Esposito MC, Cogliati M, et al. Acquired echinocandin resistance in a Candida krusei blood isolate confirmed by mutations in the FKS1 gene. New Microbiol. 2014;37(2):237–40.
Costa-de-Oliveira S, Marcos Miranda I, Silva RM, et al. FKS2 mutations associated with decreased echinocandin susceptibility of Candida glabrata following anidulafungin therapy. Antimicrob Agents Chemother. 2011;55(3):1312–4.
Jensen RH, Justesen US, Rewes A, Perlin DS, Arendrup MC. Echinocandin failure case due to a previously unreported FKS1 mutation in Candida krusei. Antimicrob Agents Chemother. 2014;58(6):3550–2.
Arendrup MC, Perlin DS, Jensen RH, et al. Differential in vivo activities of anidulafungin, caspofungin, and micafungin against Candida glabrata isolates with and without FKS resistance mutations. Antimicrob Agents Chemother. 2012;56(5):2435–42.
Vermitsky JP, Edlind TD. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a PDR1-like transcription factor. Antimicrob Agents Chemother. 2004;48(10):3773–81.
Acknowledgement
This work was supported by EraNet funding (Austrian Science Fund, Project ZFI006560/AspBIOmics).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
M. Lackner has received honoraria for invited talks by the pharmaceutical company Forest Pharmaceuticals. In the past 5 years, C. Lass-Flörl has received grant support from the Austrian Science Fund (FWF), MFF Tirol, Astellas Pharma, Gilead Sciences, Pfizer, Schering Plough, and Merck Sharp & Dohme. She has been an advisor/consultant to Gilead Sciences, Merck Sharp & Dohme, Pfizer, and Schering Plough. She has received travel/accommodation expenses from Gilead Sciences, Merck Sharp & Dohme, Pfizer, Astellas, and Schering Plough and has been paid for talks on behalf of Gilead Sciences, Merck Sharp & Dohme, Pfizer, Astellas, and Schering Plough. A. Martin-Vicente has no potential conflict of interest to state.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Current Management of Fungal Infections
Rights and permissions
About this article
Cite this article
Lackner, M., Martin-Vicente, A. & Lass-Flörl, C. Multidrug- and Cross-Resistant Candida: the Looming Threat. Curr Fungal Infect Rep 9, 23–36 (2015). https://doi.org/10.1007/s12281-014-0210-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-014-0210-1