Abstract
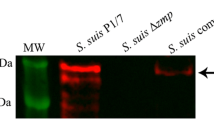
Streptococcus suis serotype 2 (S. suis 2) is an important zoonotic pathogen that presents a significant threat both to pigs and to workers in the pork industry. The initial steps of S. suis 2 pathogenesis are unclear. In this study, we found that the type II histidine triad protein HtpsC from the highly virulent Chinese isolate 05ZYH33 is structurally similar to internalin A (InlA) from Listeria monocytogenes, which plays an important role in mediating listerial invasion of epithelial cells. To determine if HtpsC and InlA function similarly, an isogenic htpsC mutant (ΔhtpsC) was generated in S. suis by homologous recombination. The htpsC deletion strain exhibited a diminished ability to adhere to and invade epithelial cells from different sources. Double immunofluorescence microscopy also revealed reduced survival of the ΔhtpsC mutant after co-cultivation with epithelium. Adhesion to epithelium and invasion by the wild type strain was inhibited by a monoclonal antibody against E-cadherin. In contrast, the htpsC-deficient mutant was unaffected by the same treatment, suggesting that E-cadherin is the host-cell receptor that interacts with HtpsC and facilitates bacterial internalization. Based on these results, we propose that HtpsC is involved in the process by which S. suis 2 penetrates host epithelial cells, and that this protein is an important virulence factor associated with cell adhesion and invasion.
Similar content being viewed by others
References
Auger, J.P., Christodoulides, M., Segura, M., Xu, J., and Gottschalk, M. 2015. Interactions of Streptococcus suis serotype 2 with human meningeal cells and astrocytes. BMC Res. Notes8, 607.
Benga, L., Goethe, R., Rohde, M., and Valentin-Weigand, P. 2010. Non-encapsulated strains reveal novel insights in invasion and survival of Streptococcus suis in epithelial cells. Cell. Microbiol.6, 867–881.
Bennett-Wood, V.R., Carapetis, J.R., and Robins-Browne, R.M. 1998. Ability of clinical isolates of group A streptococci to adhere to and invade HEp-2 epithelial cells. J. Med. Microbiol.47, 899–906.
Bierne, H., Sabet, C., Personnic, N., and Cossart, P. 2007. Internalins: a complex family of leucine-rich repeat-containing proteins in Listeria monocytogenes. Microbes Infect.9, 1156–1166.
Bober, M., Mörgelin, M., Olin, A.I., von Pawel-Rammingen, U., and Collin, M. 2011. The membrane bound LRR lipoprotein Slr, and the cell wall-anchored M1 protein from Streptococcus pyogenes both interact with type I collagen. PLoS ONE6, e20345.
Chen, C., Tang, J., Dong, W., Wang, C., Feng, Y., Wang, J., Zheng, F., Pan, X., Liu, D., Li, M., et al. 2007. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS ONE2, e315.
Eshghi, A., Gaultney, R.A., England, P., Brûlé, S., Miras, I., Sato, H., Coburn, J., Bellalou, J., Moriarty, T.J., Haouz, A., et al. 2018. An extracellular Leptospira interrogans leucine-rich repeat protein binds human E- and VE-cadherins. Cell. Microbiol.21, e12949.
Ferrando, M.L., De Greeff, A., van Rooijen, W.J.M., Stockhofe-Zurwieden, N., Nielsen, J., Wichgers Schreur, P.J., Nielsen, J., Schreur, P.J.W., Pannekoek, Y., Heuvelink, A., et al. 2015. Host-pathogen interaction at the intestinal mucosa correlates with zoonotic potential of Streptococcus suis. J. Infect. Dis.212, 95–105.
Ferrando, M.L. and Schultsz, C. 2016. A hypothetical model of host-pathogen interaction of Streptococcus suis in the gastro-intestinal tract. Gut Microbes7, 154–162.
Fittipaldi, N., Segura, M., Grenier, D., and Gottschalk, M. 2012. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol.7, 259–279.
Fouts, D.E., Matthias, M.A., Adhikarla, H., Adler, B., Amorim-Santos, L., Berg, D.E., Bulach, D., Buschiazzo, A., Chang, Y.F., Galloway, R., et al. 2016. What makes a bacterial species pathogenic?: comparative genomic analysis of the genus Leptospira. PLoS Negl. Trop. Dis.10, e0004403.
Garandeau, C., Réglier-Poupet, H., Dubail, I., Beretti, J.L., Berche, P., and Charbit, A. 2002. The sortase SrtA of Listeria monocytogenes is involved in processing of internalin and in virulence. Infect. Immun.70, 1382–1390.
Hamon, M., Bierne, H., and Cossart, P. 2006. Listeria monocytogenes a multifaceted model. Nat. Rev. Microbiol.4, 423–434.
Handfield, M., Progulske-Fox, A., and Hillman, J.D. 2005. In vivo induced genes in human diseases. Periodontology 200038, 123–134.
Lalonde, M., Segura, M., Lacouture, S., and Gottschalk, M. 2000. Interactions between Streptococcus suis serotype 2 and different epithelial cell lines. Microbiology146, 1913–1921.
Lecuit, M., Nelson, D.M., Smith, S.D., Khun, H., Huerre, M., Vacher-Lavenu, M.C., Gordon, J.I., and Cossart, P. 2004. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes role of internalin interaction with trophoblast E-cadherin. Proc. Natl. Acad. Sci. USA101, 6152–6157.
Lecuit, M., Ohayon, H., Braun, L., Mengaud, J., and Cossart, P. 1997. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect. Immun.65, 5309–5319.
Li, M., Shao, Z.Q., Guo, Y., Wang, L., Hou, T., Hu, D., Zheng, F., Tang, J., Wang, C., Feng, Y., et al. 2015. The type II histidine triad protein HtpsC is a novel adhesion with the involvement of Streptococcus suis virulence. Virulence6, 631–641.
Li, S., Song, J., Huang, H., Chen, W., Li, M., Zhao, Y., Cong, Y., Zhu, J., Rao, X., Hu, X., et al. 2013. Identification of in-vivo induced genes of Streptococcus suis serotype 2 specially expressed in infected human. Microb. Pathog.63, 8–15.
Li, J., Tan, C., Zhou, Y., Fu, S., Hu, L., Hu, J., Chen, H., and Bei, W. 2011. The two-component regulatory system CiaRH contributes to the virulence of Streptococcus suis 2. Vet. Microbiol.148, 99–104.
Li, M., Wang, C., Feng, Y., Pan, X., Cheng, G., Wang, J., Ge, J., Zheng, F., Cao, M., Dong, Y., et al. 2008. SalK/SalR, a two-component signal transduction system, is essential for full virulence of highly invasive Streptococcus suis serotype 2. PLoS ONE3, e2080.
Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods25, 402–408.
Liu, H., Zhu, S., Sun, Y., Li, N., Gu, J., Sun, C., Feng, X., Han, W., Jiang, J.X., and Lei, L. 2017. Selection of potential virulence factors contributing to Streptococcus suis serotype 2 penetration into the blood-brain barrier in an in vitro co-culture model. J. Microbiol. Biotechnol.27, 161–170.
Lun, Z.R., Wang, Q.P., Chen, X.G., Li, A.X., and Zhu, X.Q. 2007. Streptococcus suis an emerging zoonotic pathogen. Lancet Infect. Dis.7, 201–209.
Maddocks, S.E., Jenkins, R.E., Rowlands, R.S., Purdy, K.J., and Cooper, R.A. 2013. Manuka honey inhibits adhesion and invasion of medically important wound bacteria in vitro. Future Microbiol.8, 1523–1536.
Meng, F., Wu, N.H., Seitz, M., Herrler, G., and Valentin-Weigand, P. 2016. Efficient suilysin-mediated invasion and apoptosis in porcine respiratory epithelial cells after streptococcal infection under air-liquid interface conditions. Sci. Rep.6, 26748.
Miras, I., Saul, F., Nowakowski, M., Weber, P., Haouz, A., Shepard, W., and Picardeau, M. 2015. Structural characterization of a novel subfamily of leucine-rich repeat proteins from the human pathogen Leptospira interrogans. Acta Cryst.71, 1351–1359.
Ng, A.C., Eisenberg, J.M., Heath, R.J., Huett, A., Robinson, C.M., Nau, G.J., and Xavier, R.J. 2011. Human leucine-rich repeat proteins: a genome-wide bioinformatic categorization and functional analysis in innate immunity. Proc. Natl. Acad. Sci. USA108, 4631–4638.
Nielsen, H., Engelbrecht, J., Brunak, S., and von Heijne, G. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng.10, 1–6.
Normile, D. 2005. WHO probes deadliness of China’s pig-borne disease. Science309, 1308–1309.
Norton, P.M., Rolph, C., Ward, P.N., Bentley, R.W., and Leigh, J.A. 1999. Epithelial invasion and cell lysis by virulent strains of Streptococcus suis is enhanced by the presence of suilysin. FEMS Immunol. Med. Microbiol.26, 25–35.
Picardeau, M. 2017. Virulence of the zoonotic agent of leptospirosis: still terra incognita? Nat. Rev. Microbiol.15, 297–307.
Pizarro-Cerdá, J., Kühbacher, A., and Cossart, P. 2012. Entry of Listeria monocytogenes in mammalian epithelial cells: an updated view. Cold Spring Harb. Perspect. Med.2, a010009.
Schwerk, C., Papandreou, T., Schuhmann, D., Nickol, L., Borkowski, J., Steinmann, U., Quednau, N., Stump, C., Weiss C., Berger, J., et al. 2012. Polar invasion and translocation of Neisseria meningitidis and Streptococcus suis in a novel human model of the blood-cerebrospinal fluid barrier. PLoS ONE7, e30069.
Segura, M., Calzas, C., Grenier, D., and Gottschalk, M. 2016. Initial steps of the pathogenesis of the infection caused by Streptococcus suis fighting against nonspecific defenses. FEBS Lett.590, 3772–3799.
Segura, M., Fittipaldi, N., Calzas, C., and Gottschalk, M. 2017. Critical Streptococcus suis virulence factors: are they all really critical? Trends Microbiol.25, 585–599.
Seitz, M., Baums, C.G., Neis, C., Benga, L., Fulde, M., Rohde, M., Goethe, R., and Valentin-Weigand, P. 2013. Subcytolytic effects of suilysin on interaction of Streptococcus suis with epithelial cells. Vet. Microbiol.167, 584–591.
Shi, X., Ye, H., Wang, J., Li, Z., Wang, J., Chen, B., Wen, R., Hu, Q., and Feng, Y. 2016. Loss of 89K pathogenicity island in epidemic Streptococcus suis, China. Emerg. Infect. Dis.22, 1126–1127.
Sriskandan, S. and Slater, J.D. 2006. Invasive disease and toxic shock due to zoonotic Streptococcus suis an emerging infection in the east? PLoS Med.3, e187.
Staats, J.J., Feder, I., Okwumabua, O., and Chengappa, M.M. 1997. Streptococcus suis past and present. Vet. Res. Commun.21, 381–407.
Sutcliffe, I.C. and Harrington, D.J. 2002. Pattern searches for the identification of putative lipoprotein genes in Gram-positive bacterial genomes. Microbiology148, 2065–2077.
Takamatsu, D., Osaki, M., and Sekizaki, T. 2001. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid45, 101–113.
Tang, J., Wang, C., Feng, Y., Yang, W., Song, H., Chen, Z., Yu, H., Pan, X., Zhou, X., Wang, H., et al. 2006. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med.3, e151.
Valenti-Weigand, P., Benkel, P., Rohde, M., and Chhatwal, G.S. 1996. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect. Immun.64, 2467–2473.
Vanier, G., Segura, M., Friedl, P., Lacouture, S., and Gottschalk, M. 2004. Invasion of porcine brain microvascular endothelial cells by Streptococcus suis serotype 2. Infect. Immun.72, 1441–1449.
Vanier, G., Segura, M., and Gottschalk, M. 2007. Characterization of the invasion of porcine endothelial cells by Streptococcus suis serotype 2. Can. J. Vet. Res.71, 81–89.
Ye, H., Cai, M., Zhang, H., Li, Z., Wen, R., and Feng, Y. 2016. Functional definition of BirA suggests a biotin utilization pathway in the zoonotic pathogen Streptococcus suis. Sci. Rep.6, 26479.
Zheng, F., Shao, Z.Q., Hao, X., Wu, Q., Li, C., Hou, H., Hu, D., Wang, C., and Pan, X. 2018. Identification of oligopeptide-binding protein (OppA) and its role in the virulence of Streptococcus suis serotype 2. Microb. Pathog.118, 322–329.
Zipfel, C. and Felix, G. 2005. Plants and animals: a different taste for microbes? Curr. Opin. Plant Biol.8, 353–360.
Acknowledgements
This work was supported by the National Natural Science foundation of China (award 81472932), the National Key Research and Development Program of China (award 2018-YFC1602800), and the Chongqing Basic Research and Frontier Exploration Project (award cstc2019jcyj-msxmX0142).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors have no conflict of interest to report.
Additional information
Supplemental material for this article may be found at http://www.springerlink.com/content/120956.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lu, Y., Li, S., Shen, X. et al. The type II histidine triad protein HtpsC facilitates invasion of epithelial cells by highly virulent Streptococcus suis serotype 2. J Microbiol. 59, 949–957 (2021). https://doi.org/10.1007/s12275-021-1129-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-021-1129-1