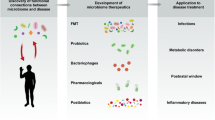
Abstract
Mucosal surfaces that line our gastrointestinal tract are continuously exposed to trillions of bacteria that form a symbiotic relationship and impact host health and disease. It is only beginning to be understood that the cross-talk between the host and microbiome involve dynamic changes in commensal bacterial population, secretion, and absorption of metabolites between the host and microbiome. As emerging evidence implicates dysbiosis of gut microbiota in the pathology and progression of various diseases such as inflammatory bowel disease, obesity, and allergy, conventional treatments that either overlook the microbiome in the mechanism of action, or eliminate vast populations of microbes via wide-spectrum antibiotics need to be reconsidered. It is also becoming clear the microbiome can influence the body’s response to therapeutic treatments for cancers. As such, targeting the microbiome as treatment has garnered much recent attention and excitement from numerous research labs and biotechnology companies. Treatments range from fecal microbial transplantation to precision-guided molecular approaches. Here, we survey recent progress in the development of innovative therapeutics that target the microbiome to treat disease, and highlight key findings in the interplay between host microbes and therapy.
Similar content being viewed by others
References
Andremont, A. 2017. Too early to recommend early fecal microbiota transplantation (FMT) in patients with severe Clostridium difficile infections (CDI), or not too early? Clin. Infect. Dis. DOI: 10.1093/cid/cix763 (in press).
Aron-Wisnewsky, J. and Clement, K. 2016. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat. Rev. Nephrol. 12, 169–181.
Bagdasarian, N., Rao, K., and Malani, P.N. 2015. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA 313, 398–408.
Belkaid, Y. and Naik, S. 2013. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 14, 646–653.
Bennet, J.D. and Brinkman, M. 1989. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet 1, 164.
Bikard, D. and Barrangou, R. 2017. Using CRISPR-Cas systems as antimicrobials. Curr. Opin. Microbiol. 37, 155–160.
Bikard, D., Euler, C.W., Jiang, W., Nussenzweig, P.M., Goldberg, G.W., Duportet, X., Fischetti, V.A., and Marraffini, L.A. 2014. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol. 32, 1146–1150.
Borody, T.J., Warren, E.F., Leis, S., Surace, R., and Ashman, O. 2003. Treatment of ulcerative colitis using fecal bacteriotherapy. J. Clin. Gastroenterol. 37, 42–47.
Brown, S.L., Riehl, T.E., Walker, M.R., Geske, M.J., Doherty, J.M., Stenson, W.F., and Stappenbeck, T.S. 2007. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J. Clin. Invest. 117, 258–269.
Browne, A.S. and Kelly, C.R. 2017. Fecal transplant in inflammatory bowel disease. Gastroenterol. Clin. North Am. 46, 825–837.
Colman, R.J. and Rubin, D.T. 2014. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J. Crohns Colitis 8, 1569–1581.
DeGruttola, A.K., Low, D., Mizoguchi, A., and Mizoguchi, E. 2016. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 22, 1137–1150.
Donaldson, G.P., Lee, S.M., and Mazmanian, S.K. 2016. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32.
Dubberke, E.R., Mullane, K.M., Gerding, D.N., Lee, C.H., Louie, T.J., Guthertz, H., and Jones, C. 2016. Clearance of vancomycin-resistant Enterococcus concomitant with administration of a microbiota-based drug targeted at recurrent Clostridium difficile infection. Open Forum Infect. Dis. 3, ofw133.
Duncan, S.H., Scott, K.P., Ramsay, A.G., Harmsen, H.J., Welling, G.W., Stewart, C.S., and Flint, H.J. 2003. Effects of alternative dietary substrates on competition between human colonic bacteria in an anaerobic fermentor system. Appl. Environ. Microbiol. 69, 1136–1142.
Farber, J., Illiger, S., Berger, F., Gartner, B., von Muller, L., Lohmann, C.H., Bauer, K., Grabau, C., Zibolka, S., Schluter, D., et al. 2017. Management of a cluster of Clostridium difficile infections among patients with osteoarticular infections. Antimicrob. Resist. Infect. Control 6, 22.
Flint, H.J., Scott, K.P., Duncan, S.H., Louis, P., and Forano, E. 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3, 289–306.
Furusawa, Y., Obata, Y., Fukuda, S., Endo, T.A., Nakato, G., Takahashi, D., Nakanishi, Y., Uetake, C., Kato, K., Kato, T., et al. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450.
Ge, P., Scholl, D., Leiman, P.G., Yu, X., Miller, J.F., and Zhou, Z.H. 2015. Atomic structures of a bactericidal contractile nanotube in its pre-and postcontraction states. Nat. Struct. Mol. Biol. 22, 377–382.
Gebhart, D., Lok, S., Clare, S., Tomas, M., Stares, M., Scholl, D., Donskey, C.J., Lawley, T.D., and Govoni, G.R. 2015. A modified R-type bacteriocin specifically targeting Clostridium difficile prevents colonization of mice without affecting gut microbiota diversity. MBio 6, e02368–14.
Gebhart, D., Williams, S.R., Bishop-Lilly, K.A., Govoni, G.R., Willner, K.M., Butani, A., Sozhamannan, S., Martin, D., Fortier, L.C., and Scholl, D. 2012. Novel high-molecular-weight, R-type bacteriocins of Clostridium difficile. J. Bacteriol. 194, 6240–6247.
Geirnaert, A., Calatayud, M., Grootaert, C., Laukens, D., Devriese, S., Smagghe, G., De Vos, M., Boon, N., and Van de Wiele, T. 2017. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 7, 11450.
Gopalakrishnan, V., Spencer, C.N., Nezi, L., Reuben, A., Andrews, M.C., Karpinets, T.V., Prieto, P.A., Vicente, D., Hoffman, K., Wei, S.C., et al. 2018. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103.
Guo, L., McLean, J.S., Yang, Y., Eckert, R., Kaplan, C.W., Kyme, P., Sheikh, O., Varnum, B., Lux, R., Shi, W., et al. 2015. Precisionguided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. USA 112, 7569–7574.
Haurwitz, R.E., Jinek, M., Wiedenheft, B., Zhou, K., and Doudna, J.A. 2010. Sequence-and structure-specific RNA processing by a CRISPR endonuclease. Science 329, 1355–1358.
Hemarajata, P. and Versalovic, J. 2013. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap. Adv. Gastroenterol. 6, 39–51.
Kelly, C.R., Kahn, S., Kashyap, P., Laine, L., Rubin, D., Atreja, A., Moore, T., and Wu, G. 2015. Update on fecal microbiota transplantation 2015: Indications, methodologies, mechanisms, and outlook. Gastroenterology 149, 223–237.
Khanna, S. 2018. Microbiota replacement therapies: Innovation in gastrointestinal care. Clin. Pharmacol. Ther. 103, 102–111.
Khanna, S. and Raffals, L.E. 2017. The microbiome in Crohn’s Disease: Role in pathogenesis and role of microbiome replacement therapies. Gastroenterol. Clin. North Am. 46, 481–492.
Kirk, J.A., Gebhart, D., Buckley, A.M., Lok, S., Scholl, D., Douce, G.R., Govoni, G.R., and Fagan, R.P. 2017. New class of precision antimicrobials redefines role of Clostridium difficile S-layer in virulence and viability. Sci. Transl. Med. 9, eaah6813.
Knight, D.R., Elliott, B., Chang, B.J., Perkins, T.T., and Riley, T.V. 2015. Diversity and evolution in the genome of Clostridium difficile. Clin. Microbiol. Rev. 28, 721–741.
LeBlanc, J.G., Milani, C., de Giori, G.S., Sesma, F., van Sinderen, D., and Ventura, M. 2013. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24, 160–168.
Lopez, C.A., Kingsbury, D.D., Velazquez, E.M., and Baumler, A.J. 2014. Collateral damage: microbiota-derived metabolites and immune function in the antibiotic era. Cell Host Microbe 16, 156–163.
Mathewson, N.D., Jenq, R., Mathew, A.V., Koenigsknecht, M., Hanash, A., Toubai, T., Oravecz-Wilson, K., Wu, S.R., Sun, Y., Rossi, C., et al. 2016. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 17, 505–513.
Matson, V., Fessler, J., Bao, R., Chongsuwat, T., Zha, Y., Alegre, M.L., Luke, J.J., and Gajewski, T.F. 2018. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108.
Moayyedi, P., Surette, M.G., Kim, P.T., Libertucci, J., Wolfe, M., Onischi, C., Armstrong, D., Marshall, J.K., Kassam, Z., Reinisch, W., et al. 2015. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109.e6.
Neish, A.S. 2009. Microbes in gastrointestinal health and disease. Gastroenterology 136, 65–80.
Nishida, A., Inoue, R., Inatomi, O., Bamba, S., Naito, Y., and Andoh, A. 2017. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 11, 1–10.
Paramsothy, S., Kamm, M.A., Kaakoush, N.O., Walsh, A.J., van den Bogaerde, J., Samuel, D., Leong, R.W.L., Connor, S., Ng, W., Paramsothy, R., et al. 2017. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 389, 1218–1228.
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S., and Medzhitov, R. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241.
Rossen, N.G., MacDonald, J.K., de Vries, E.M., D’Haens, G.R., de Vos, W.M., Zoetendal, E.G., and Ponsioen, C.Y. 2015. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J. Gastroenterol. 21, 5359–5371.
Round, J.L. and Mazmanian, S.K. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 313–323.
Routy, B., Le Chatelier, E., Derosa, L., Duong, C.P.M., Alou, M.T., Daillere, R., Fluckiger, A., Messaoudene, M., Rauber, C., Roberti, M.P., et al. 2018. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97.
Schwan, A., Sjolin, S., Trottestam, U., and Aronsson, B. 1983. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet 2, 845.
Suskind, D.L., Brittnacher, M.J., Wahbeh, G., Shaffer, M.L., Hayden, H.S., Qin, X., Singh, N., Damman, C.J., Hager, K.R., Nielson, H., et al. 2015. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm. Bowel Dis. 21, 556–563.
Talbot, A.M. 2017. Vedanta biosciences announces initiation of phase 1a/1b trial for new drug class of rationally-defined bacterial consortia derived from the human microbiome. Business Wire. PureTech Health. Available at https://www.businesswire. com/news/home/20171206006422/en/Vedanta-Biosciences-An nounces-Initiation-Phase-1a1b-Trial (accessed December 07, 2017).
Vindigni, S.M. and Surawicz, C.M. 2017. Fecal microbiota transplantation. Gastroenterol. Clin. North Am. 46, 171–185.
Yosef, I., Manor, M., Kiro, R., and Qimron, U. 2015. Temperate and lytic bacteriophages programmed to sensitize and kill antibioticresistant bacteria. Proc. Natl. Acad. Sci. USA 112, 7267–7272.
Zhang, F., Wen, Y., and Guo, X. 2014. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum. Mol. Genet. 23, R40–R46.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, J.A., Chinnapen, D.J. Targeting friend and foe: Emerging therapeutics in the age of gut microbiome and disease. J Microbiol. 56, 183–188 (2018). https://doi.org/10.1007/s12275-018-8037-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-018-8037-z