Abstract
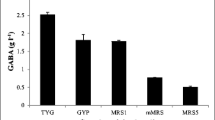
Succinic acid is one of the platform compounds and its production via natural feedstocks has drawn worldwide concerns. To evaluate the inhibitory effects of fermentation products on the growth of Actinobacillus succinogenes 130ZT and Escherichia coli NZN111, AFP111, BL21, fermentations with addition of individual products in medium were carried out. The cell growth was inhibited when the concentrations of formate, acetate, lactate, and succinate were at range of 8.8–17.6 g/L, 10–40 g/L, 9–18 g/L, and 10–80 g/L, respectively. For these two species of bacteria, E. coli was more resistant to acid products than A. succinogenes, while both endured succinate rather than by-products. As a result of end product inhibition, succinate production yield by A. succinogenes decreased from 1.11 to 0.49 g/g glucose. Logistic and Monod mathematical models were presented to simulate the inhibition kinetics. The Logistic model was found more suitable for describing the overall synergistic inhibitory effects.
Similar content being viewed by others
References
Andersson, C., D. Hodge, K.A. Berglund, and U. Rova. 2007. Effect of different carbon sources on the production of succinic acid using metabolically engineered Escherichia coli. Biotechnol. Progr. 23, 381–388.
Chen, C.C. and L.K. Ju. 2002. Coupled lactic acid fermentation and adsorption. Appl. Microbiol. Biotechnol. 59, 170–174.
Colin, T., A. Bories, and G. Moulin. 2000. Inhibition of Clostridium butyricum by 1, 3-propanediol and diols during glycerol fermentation. Appl. Microbiol. Biotechnol. 54, 201–205.
Corona-González, R.I., A. Bories, V. González-Álvarez, and C. Pelayo-Ortiz. 2008. Kinetic study of succinic acid production by Actinobacillus succinogenes ZT-130. Process Biochem. 43, 1047–1053.
Delhomme, C., D. Weuster-Botz, and F.E. Kühn. 2009. Succinic acid from renewable resources as a C4 building-block chemical-a review of the catalytic possibilities in aqueous media. Green Chem. 11, 13–26.
Goncalves, L.M.D., A. Ramos, A.S. Almedia, A.M.R.B. Xavier, and M.J.T. Carrondo. 1997. Elucidation of the mechanism of lactic acid growth inhibition and production in batch cultures of Lactobacillus rhamnosus. Appl. Microbiol. Biotechnol. 48, 346–350.
Guettler, M.V., M.K. Jain, and D. Rumler. 1996. Method for making succinic acid, bacterial variants for use in the process, and methods for obtaining variants. US Patent 5,573,931.
Guettler, M.V., D. RumLer, and M.K. Jain. 1999. Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen. Int. J. Syst. Bacteriol. 49, 207–216.
Kacena, M.A., G.A. Merrell, B. Manfredi, E.E. Smith, D.M. Klaus, and P. Todd. 1999. Bacterial growth in space flight: logistic growth curve parameters for Escherichia coli and Bacillus subtilis. Appl. Microbiol. Biotechnol. 51, 229–234.
Kang, H.C., Y.H. Park, and S.J. Go. 2003. Growth inhibition of a phytopathogenic fungus, Colletotrichum species by acetic acid. Microbiol. Res. 158, 321–326.
Lee, S.Y., J.M. Kim, H. Song, J.W. Lee, T.Y. Kim, and Y.S. Jang. 2008. From genome sequence to integrated bioprocess for succinic acid production by Mannheimia succiniciproducens. Appl. Microbiol. Biotechnol. 79, 11–22.
Lee, S.L., D.Y. Lee, T.Y. Kim, B.H. Kim, J.W. Lee, and S.Y. Lee. 2005. Metabolic engineering of Escherichia coli for enhanced production of succinic acid, based on genome comparison and in silico gene knockout simulation. Appl. Environ. Microbiol. 71, 7880–7887.
Lee, S.J., H. Song, and S.Y. Lee. 2006. Genome-based metabolic engineering of Mannheimia succiniciproducens for succinic acid production. Appl. Environ. Microbiol. 72, 1939–1948.
Lin, C.S.K., C.Y. Du, A. Koutinas, R. Wang, and C. Webb. 2008. Substrate and product inhibition kinetics in succinic acid production by Actinobacillus succinogenes. Biochem. Eng. J. 41, 128–135.
Luque, R., C.S.K. Lin, C.Y. Du, D.J. Macquarrie, A. Koutinas, R.H. Wang, C. Webb, and J.H. Clark. 2009. Chemical transformations of succinic acid recovered from fermentation broths by a novel direct vacuum distillation-crystallisation method. Green Chem. 11, 193–200.
McKinlay, J.B. and C. Vieille. 2008. 13C-metabolic flux analysis of Actinobacillus succinogenes fermentative metabolism at different NaHCO3 and H2 concentrations. Metab. Eng. 10, 55–68.
McKinlay, J.B., C. Vieille, and J.G. Zeikus. 2007. Prospects for a bio-based succinate industry. Appl. Microbiol. Biotechnol. 76, 727–740.
Okino, S., R. Noburyu, M. Suda, T. Jojima, M. Inui, and H. Yukawa. 2008. An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain. Appl. Microbiol. Biotechnol. 81, 459–464.
Oliva, J., M. Negro, F. Sáez, I. Ballesteros, P. Manzanares, A. González, and M. Ballesteros. 2006. Effects of acetic acid, furfural and catechol combinations on ethanol fermentation of Kluyveromyces marxianus. Process Biochem. 41, 1223–1228.
Park, D.H. and J.G. Zeikus. 1999. Utilization of electrically reduced neutral red by Actinobacillus succinogenes: physiological function of neutral red in membrane-driven fumarate reduction and energy conservation. J. Bacteriol. 181, 2403–2410.
Peleg, M., M.G. Corradini, and M.D. Normand. 2007. The logistic (Verhulst) model for sigmoid microbial growth curves revisited. Food Res. Int. 40, 808–818.
Phue, J.N. and J. Shiloach. 2005. Impact of dissolved oxygen concentration on acetate accumulation and physiology of E. coli BL21 evaluating transcription levels of key genes at different dissolved oxygen conditions. Metab. Eng. 7, 353–363.
Sánchez, A.M., G.N. Bennett, and K.Y. San. 2005. Novel pathway engineering design of the anaerobic central metabolic pathway in Escherichia coli to increase succinate yield and productivity. Metab. Eng. 7, 229–239.
Song, H., S.H. Jang, J.M. Park, and S.Y. Lee. 2008. Modeling of batch fermentation kinetics for succinic acid production by Mannheimia succiniciproducens. Biochem. Eng. J. 40, 107–115.
Urbance, S.E., A.L. Pometto, A.A. DiSpirito, and Y. Denli. 2004. Evaluation of succinic acid continuous and repeat-batch biofilm fermentation by Actinobacillus succinogenes using plastic composite support bioreactors. Appl. Microbiol. Biotechnol. 65, 664–670.
Vemuri, G.N., M.A. Eiteman, and E. Altman. 2002. Succinate production in dual-phase Escherichia coli fermentations depends on the time of transition from aerobic to anaerobic conditions. J. Ind. Microbiol. Biotechnol. 28, 325–332.
Wee, Y.J., J.S. Yun, K.H. Kang, and H.W. Ryu. 2002. Continuous production of succinic acid by a fumarate-reducing bacterium immobilized in a hollow-fiber bioreactor. Appl. Biochem. Biotechnol. 98–100, 1093–1104.
Yang, X.P. and G.T. Tsao. 1994. Mathematical modeling of inhibition kinetics in acetone-butanol fermentation by Clostridium acetobutylicum. Biotechnol. Progr. 10, 532–538.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Q., Wang, D., Wu, Y. et al. Kinetic evaluation of products inhibition to succinic acid producers Escherichia coli NZN111, AFP111, BL21, and Actinobacillus succinogenes 130ZT . J Microbiol. 48, 290–296 (2010). https://doi.org/10.1007/s12275-010-9262-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-010-9262-2