Abstract
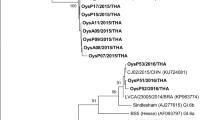
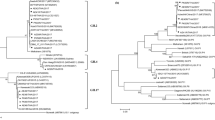
Noroviruses (NoV) are the key cause of acute epidemic gastroenteritis, and oysters harvested from NoV-polluted sea areas are considered as the significant vectors of viral transmission. To improve NoV detection from oyster using nested reverse transcription-polymerase chain reaction (RT-PCR), we evaluated the sensitivity and specificity of previously published primer pairs and the efficiency of different RNA extraction procedures. Among the primer pairs used for RT-PCR, the sensitivity of GIF1/GIR1-GIF2/GIR1 and GIIF1/GIIR1-GIIF2/GIIR1 was higher than that of other primer pairs used in nested RT-PCR for the detection of NoV genogroup I (NoV GI) and NoV GII from both NoV-positive stool suspension and NoV-seeded oyster concentrates, respectively; the resulting products showed neither unspecific bands in the positive samples nor false-positive bands in the negative controls. The extraction of NoV RNA from oyster samples using a QIAamp® Viral RNA Mini kit with a QIAshredder™ Homogenizer pretreatment afforded more efficient recovery (mean recovery for NoV GI and GII, 6.4%) and the procedure was less time consuming (<30 min) than most other RNA extraction procedures. The results of RNA extraction procedure and primer pairs evaluated by nested RT-PCR assay in this study can be useful for monitoring NoV contamination in oysters, which is an indicator of possible public health risks.
Similar content being viewed by others
References
Ando, T., J.S. Noel, and R.L. Fankhauser. 2000. Genetic classification of ‘Norwalk-like viruses’. J. Infect. Dis. 181, S336–S348.
Boxman, I.L.A., J.J.H.C. Tilburg, N.A.J.M. te Loeke, H. Vennema, K. Jonker, E. de Boer, and M. Koopmans. 2006. Detection of noroviruses in shellfish in the Netherlands. Int. J. Food Microbiol. 108, 391–396.
Bull, R.A., E.T.V. Tu, C.J. McIver, W.D. Rawlinson, and P.A. White. 2006. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J. Clin. Microbiol. 44, 327–333.
Choo, Y.J. and S.J. Kim. 2006. Detection of human adenoviruses and enteroviruses in Korean oysters using cell culture, integrated cell culture-PCR, and direct PCR. J. Microbiol. 44, 162–170.
Doyle, T.J., L. Stark, R. Hammond, and R.S. Hopkins. 2009. Outbreaks of noroviral gastroenteritis in Florida, 2006–2007. Epidemiol. Infect. 137, 617–625.
Duizer, E., K.J. Schwab, F.H. Neill, R.M. Atmar, M.P.G. Koopmans, and M. K. Estes. 2004. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 85, 79–87.
Gallimore, C.I., J.S. Cheesbrough, K. Lamden, C. Bingham, and J.J. Gray. 2005. Multiple norovirus genotypes characterised from an oyster-associated outbreak of gastroenteritis. Int. J. Food Microbiol. 103, 323–330.
Häfliger, D., M. Gilgen, J. Lüthy, and P. Hübner. 1997. Seminested RT-PCR systems for small round structured viruses and detection of enteric viruses in seafood. Int. J. Food Microbiol. 37, 27–36.
Huppatz, C., S.A. Munnoch, T. Worgan, T.D. Merritt, C. Dalton, P.M. Kelly, and D.N. Durrheim. 2008. A norovirus outbreak associated with consumption of NSW oysters: implications for quality assurance systems. Commun. Dis. Intell. 32, 88–91.
Kageyama, T., S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F.B. Hoshino, N. Takeda, and K. Katayama. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41, 1548–1557.
Kageyama, T., M. Shinohara, K. Uchida, S. Fukushi, F.B. Hoshino, S. Kojima, R. Takai, T. Oka, N. Takeda, and K. Katayama. 2004. Coexistence of multiple genotypes, including newly identified genotypes, in outbreaks of gastroenteritis due to norovirus in Japan. J. Clin. Microbiol. 42, 2988–2995.
Kim, S.H., D.S. Cheon, J.H. Kim, D.H. Lee, W.H. Jheong, Y.J. Heo, H.M. Chung, Y. Jee, and J.S. Lee. 2005. Outbreaks of gastroenteritis that occurred during school excursions in Korea were associated with several waterborne strains of norovirus. J. Clin. Microbiol. 43, 4836–4839.
La Rosa, G., S. Fontana, A. Di Grazia, M. Iaconelli, M. Pourshaban, and M. Muscillo. 2007. Molecular identification and genetic analysis of norovirus genogroups I and II in water environments: Comparative analysis of different reverse transcription-PCR assays. Appl. Environ. Microbiol. 73, 4152–4161.
Mendez, I.I., L.L. Hermann, P.R. Hazelton, and K.M. Coombs. 2000. A comparative analysis of Freon substitutes in the purification of reovirus and calicivirus. J. Virol. Methods 90, 59–67.
Mounts, A.W., T. Ando, M. Koopmans, J.S. Bresee, J. Noel, and R.I. Glass. 2000. Cold weather seasonality of gastroenteritis associated with Norwalk-like viruses. J. Infect. Dis. 181, S284–S287.
Mullendore, J.L., M.D. Sobsey, and Y.C. Shieh. 2001. Improved method for the recovery of hepatitis A virus from oysters. J. Virol. Methods 94, 25–35.
Myrmel, M., E.M.M. Berg, E. Rimstad, and B. Grinde. 2004. Detection of enteric viruses in shellfish from the Norwegian coast. Appl. Environ. Microbiol. 70, 2678–2684.
Naitou, H. and T. Morita. 2001. Selection of more appropriate PCR primer pairs for improved efficiency in detecting Norwalk-like virus (NLV) RNA. J. Gen. Appl. Microbiol. 47, 241–246.
Nappier, S.P., T.K. Graczyk, and K.J. Schwab. 2008. Bioaccumulation, retention, and depuration of enteric viruses by Crassostrea virginica and Crassostrea ariakensis oysters. Appl. Environ. Microbiol. 74, 6825–6831.
Nishida, T., H. Kimura, M. Saitoh, M. Shinohara, M. Kato, S. Fukuda, T. Munemura, T. Mikami, A. Kawamoto, M. Akiyama, Y. Kato, K. Nishi, K. Kozawa, and O. Nishio. 2003. Detection, quantitation, and phylogenetic analysis of noroviruses in Japanese oysters. Appl. Environ. Microbiol. 69, 5782–5786.
Nishida, T., O. Nishio, M. Kato, T. Chuma, H. Kato, H. Iwata, and H. Kimura. 2007. Genotyping and quantitation of noroviruses in oysters from two distinct sea areas in Japan. Microbiol. Immunol. 51, 177–184.
Okada, M., T. Ogawa, I. Kaiho, and K. Shinozaki. 2005. Genetic analysis of noroviruses in Chiba prefecture, Japan, between 1999 and 2004. J. Clin. Microbiol. 43, 4391–4401.
Sair, A.I., D.H. D’souza, C.L. Moe, and L.A. Jaykus. 2002. Improved detection of human enteric viruses in foods by RT-PCR. J. Virol. Methods 100, 57–69.
Saito, H., S. Saito, K. Kamada, S. Harata, H. Sato, M. Morita, and Y. Miyajima. 1998. Application of RT-PCR designed from the sequence of the local SRSV strain to the screening in viral gastroenteritis outbreaks. Microbiol. Immunol. 42, 439–446.
Sala, M.R., C. Arias, A. Domínguez, R. Bartolomé, and J.M. Muntada. 2009. Foodborne outbreak of gastroenteritis due to Norovirus and Vibrio parahaemolyticus. Epidemiol. Infect. 137, 626–629.
Schultz, A.C., P. Saadbye, J. Hoorfar, and B. Nørrung. 2007. Comparison of methods for detection of norovirus in oysters. Int. J. Food Microbiol. 114, 352–356.
Straub, T.M., K.H. Zu Bentrup, P. Orosz-Coghlan, A. Dohnalkova, B.K. Mayer, R.A. Bartholomew, C.O. Valdez, C.J. Bruckner-Lea, C.P. Gerba, M. Abbaszadegan, and C.A. Nickerson. 2007. In vitro cell culture infectivity assay for human noroviruses. Emerg. Infect. Dis. 13, 396–403.
Sugiyama, A., K. Nishi, T. Yano, Y. Nakano, M. Fukuta, Y. Iwade, A. Yamauchi, K. Kawada, O. Nakayama, N.H. Kumazawa, T. Nakano, T. Ihara, and H. Kamiya. 2002. Contamination of oyster sea farm with the Norwalk virus: mechanisms and control. Nippon Rinsho. 60, 1214–1221.
Ueki, Y., D. Sano, T. Watanabe, K. Akiyama, and T. Omura. 2005. Norovirus pathway in water environment estimated by genetic analysis of strains from patients of gastroenteritis, sewage, treated wastewater, river water and oysters. Water Res. 39, 4271–4280.
U.S. Food and Drug Administration. 1992. Bacteriological analytical manual. AOAC International, Arlington, VA, USA.
Vennema, H., E. De Bruin, and M. Koopmans. 2002. Rational optimization of generic primers used for Norwalk-like virus detection by reverse transcriptase polymerase chain reaction. J. Clin. Virol. 25, 233–235.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lee, C., Cheong, S., Lee, HJ. et al. Evaluation of the sensitivity and specificity of primer pairs and the efficiency of RNA extraction procedures to improve noroviral detection from oysters by nested reverse transcription-polymerase chain reaction. J Microbiol. 48, 586–593 (2010). https://doi.org/10.1007/s12275-010-0047-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-010-0047-4