Abstract
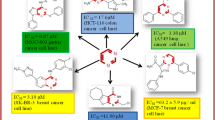
Chalcone and chromene motifs are synthetic or naturally occurring scaffolds with significant cytotoxic profile. Two types of novel regioisomeric chromene-chalcone hybrids, namely 1-(6-chloro or 6-methoxy-2H-chromen-3-yl)-3-phenylprop-2-en-1-one (Type A) and 3-(6-chloro or 6-methoxy-2H-chromen-3-yl)-1-phenylprop-2-en-1-one (Type B), both with different substituents on the phenyl ring attached to propenone linkage, have been evaluated for their cytotoxic activity against breast cancer cell lines (MCF-7 and MDA-MB-231). The results indicate that type A of chromene-chalcones demonstrated better cytotoxic profile than type B especially in MDA-MB-231 cell line. In addition, the growth inhibitory activity of most of the target compounds is higher than Etoposide as a reference drug. QSAR analysis of these novel compounds demonstrated that topological and geometrical parameters are among the important descriptors that influence the cytotoxic activity profile of compounds.
Similar content being viewed by others
References
Akbarzadeh, T., Rafinejad, A., Mollaghasem, J. M., Safavi, M., Fallah-Tafti, A., Pordeli, M., Ardestani, S. K., Shafiee, A., and Foroumadi, A., 2-Amino-3-cyano-4-(5-arylisoxazol-3-yl)-4H-chromenes: Synthesis and in vitro cytotoxic activity. Arch. Pharm. (Weinheim), 345, 386–392 (2012).
Akihisa, T., Tokuda, H., Hasegawa, D., Ukiya, M., Kimura, Y., Enjo, F., Suzuki, T., and Nishino, H., Chalcones and other compounds from the exudates of Angelica keiskei and their cancer chemopreventive effects. J. Nat. Prod., 69, 38–42 (2006).
Alizadeh, B. H., Foroumadi, A., Emami, S., Khoobi, M., Panah, F., Ardestani, S. K., and Shafiee, A., Isochaihulactone analogues: synthesis and anti-proliferative activity of novel dibenzylbutyrolactones. Eur. J. Med. Chem., 45, 5979–5984 (2010).
Belluti, F., Fontana, G., Dal, B. O. L., Carenini, N., Giommarelli, C., and Zunino, F., Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg. Med. Chem., 18, 3543–3550 (2010).
Bois, F., Boumendjel, A., Mariotte, A. M., Conseil, G., and Di Petro, A., Synthesis and biological activity of 4-alkoxy chalcones: potential hydrophobic modulators of P-glycoprotein-mediated multidrug resistance. Bioorg. Med. Chem., 7, 2691–2695 (1999).
Dimmock, J. R., Elias, D. W., Beazely, M. A., and Kandepu, N. M., Bioactivities of chalcones. Curr. Med. Chem. 6, 1125–1149 (1999).
Dimmock, J. R., Kandepu, N. M., Hetherington, M., Quail, J. W., Pugazhenthi, U., Sudom, A. M., Chamankhah, M., Rose, P., Pass, E., Allen, T. M., Halleran, S., Szydlowski, J., Mutus, B., Tannous, M., Manavathu, E. K., Myers, T. G., Clercq, E. D., and Balzarini, B., Cytotoxic activities of mannich bases of chalcones and related compounds. J. Med. Chem., 4, 1014–1026 (1998).
Foroumadi, A., Emami, S., Sorkhi, M., Nakhjiri, M., Nazarian, Z., Heydari, S., Ardestani, S. K., Poorrajab, F., and Shafiee, A., Chromene-based synthetic chalcones as potent antileishmanial agents: Synthesis and biological activity. Chem. Biol. Drug. Des., 75, 590–596 (2010).
Frisch, M. J., Trucks, M. J., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Zakrzewski, V. G., Montgomery, J. A., Stratmann, J. R., Burant, J. C., Dapprich, S., Millam, J. M., Daniels, A. D., Kudin, K. N., Strain, M. C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G. A., Ayala, P. Y., Cui, Q., Morokuma, K., Malick, D. K., Rabuck, A. D., Raghavachari, K., Foresman, J. B., Cioslowski, J., Ortiz, J. V., Baboul, A. G., Stefanov, B. B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R. L., Fox, D. J., Keith, T., Al-Laham, M. A., Peng, C. Y., Nanayakkara, A., Gonzalez, C., Challacombe, M., Gill, P. M. W., Johnson, B., Chen, W., Wong, M. W., Andres, J. L., Gonzalez, C., Head-Gordon, M., Replogle, E. S., and Pople, J. A., Gaussian 98, Revision A.7, Gaussian, Inc., Pittsburgh P. A. (1998).
Go, M. L., Wu, X., and Liu, X. L., Chalcones: an update on cytotoxic and chemopretective properties. Curr. Med. Chem., 12, 483–499 (2005).
Gunatilaka, A. L., Kingston, D. G. I., and Johnson, R. K., Mechanism-based isolation and structures of some anticancer active natural products. Pure Appl. Chem., 66, 2219–2222 (1994).
Hadfield, J. A., Ducki, S., Hirst, N., and McGown, AT., Tubulin and microtubules as target for anticancer drugs. Prog. Cell Cycle Res., 5, 309–325 (2003).
Heo, S. J., Kim, K. N., Yoon, W. J., Oha, C., Choi, Y. U., Affan, A., Lee, Y. J., Lee, H. S., and Kang, D. H., Chromene induces apoptosis via caspase-3 activation in human leukemia HL-60 cells. Food Chem. Toxicol., 49, 1998–2004 (2011).
Huang, W., Ding, Y., Miao, Y., Liu, M. Z., Li, Y., and Yang, G. F., Synthesis and antitumor activity of novel dithiocarbamate substituted chromones. Eur. J. Med. Chem., 44, 3687–3696 (2009).
Hughes, G. K., Lahey, F. N., Price, J. R., and Webb, L. J., Alkaloids of the Australian Rutaceae. Nature, 162, 223–224 (1948).
Lawrence, N. J., McGown, A. T., Ducki, S., and Hadfield, J. A., The interaction of chalcones with tubulin. Anticancer Drug Des., 15, 135–141 (2000).
Lawrence, N. J. and McGown, A. T., The chemistry and biology of antimitotic chalcones and related enone systems. Curr. Pharm. Des., 11, 1679–1693 (2005).
Liu, M., Wilairat, P., and Croft, S. L., Structure-activity relationships of antileishmanial and antimalarial chalcones. Bioorg. Med. Chem., 11, 2729–2738 (2003).
Liu, Q., Wang, Y. F., Chen, R. J., Zhang, M. Y., Wang, Y. F., Yang, C. R., and Zhang, Y. J., Anti-Coxsackie virus B3 norsesquiterpenoids from the roots of Phyllanthus emblica. J. Nat. Prod., 72, 969–972 (2009).
Liu, X. L., Tee, H. W., and Go, M. L., Functionalized chalcones as selective inhibitors of P-glycoprotein and breast cancer resistance protein. Bioorg. Med. Chem., 16, 171–180 (2008).
Mager, H., The problem of multicollinearity in Hansch-type quantitative structure-activity relationships (QSAR) and in chemical linear free energy relationships (LFER). Pharmazie, 38, 120–128 (1983).
Mahmoodi, M., Aliabadi, A., Emami, S., Safavi, M., Rajabalian, S., Mohagheghi, M. A., Khoshzaban, A., Samzadeh-Kermani, A., Lamei, N., Shafiee, A., and Foroumadi, A., Synthesis and in-vitro cytotoxicity of poly-functionalized 4-(2-arylthiazol-4-yl)-4H-chromenes. Arch. Pharm. (Weinheim), 343, 411–416 (2010).
Mao, W., Wang, T., Zeng, H., Wang, Z., Chen, J., and Shen, J., Synthesis and evaluation of novel substituted 5-hydroxycoumarin and pyranocoumarin derivatives exhibiting significant antiproliferative activity against breast cancer cell lines. Bioorg. Med. Chem. Lett., 19, 4570–4573 (2009).
Mayur, Y. C., Peters, G. J., Prasad, V. V., Lemo, C., and Sathish, N. K., Design of new drug molecules to be used in reversing multidrug resistance in cancer cells. Curr. Cancer Drug Targets, 9, 298–306 (2009).
Mosmann, T., Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immun. Methods, 65, 55–63 (1983).
Nakatani, N., Ichimaru, M., Moriyasu, M., and Kato, A., Induction of apoptosis in human promyelocytic leukemia cell line HL-60 by C-benzylated dihydrochalcones, uvaretin, isouvaretin and diuvaretin. Biol. Pharm. Bull., 28, 83–86 (2005).
Nazarian, Z., Emami, S., Heydari, S., Ardestani, S. K., Nakhjiri, M., Poorrajab, F., and Shafiee, A., Novel antileishmanial chalconoids: Synthesis and biological activity of 1- or 3-(6-chloro-2H-chromen-3-yl)propen-1-ones. Eur. J. Med. Chem., 45, 1424–1429 (2010).
Nowakowska, Z., A review of antiinfective and anti-inflammatory chalcones. Eur. J. Med. Chem., 42, 125–137 (2007).
Park, E. J., Park, H. R., Lee, J. S., and Kim, J., Lichochalcone A: an inducer of cell differentiation and cytotoxic agent from Pogostemon cablin. Planta Med., 64, 464–466 (1998).
Patil, C. B., Mahajan, S. K., and Katti, S. A., Chalcone: A versatile molecule. J. Pharm. Sci. Res., 1, 11–22 (2009).
Reddy, M. V., Su, C. R., Chiou, W. F., Liu, Y. N., Chen, R. Y., Bastow, K. F., Lee, K. H., and Wu, T. S., Design, synthesis, and biological evaluation of Mannich bases of heterocyclic chalcone analogs as cytotoxic agents. Bioorg. Med. Chem., 16, 7358–7370 (2008).
Reddy, M. V., Shen, Y. C., Yang, J. S., Hwang, T. L., Bastow, K. F., Qian, K., Lee, K. H., and Wu, T. S., New bichalcone analogs as NF-κB inhibitors and as cytotoxic agents inducing Fas/CD95-dependent apoptosis. Bioorg. Med. Chem., 19, 1895–1906 (2011).
Rojas, J., Paya, M., Dominguez, J. N., and Ferrandiz, M. L., The synthesis and effect of fluorinated chalcone derivatives on nitric oxide production. Bioorg. Med. Chem. Lett., 12, 1951–1954 (2002).
Rozmer, Z., Berki, T., and Perjési, P., Different effects of two cyclic chalcone analogues on cell cycle of Jurkat T cells. Toxicol. In Vitro, 20, 1354–1362 (2006).
Sashidhara, K. V., Kumar, A., Kumar, M., Sarkar, J., and Sinha, S., Synthesis and in vitro evaluation of novel coumarin-chalcone hybrids as potential anticancer agents Bioorg. Med. Chem. Lett., 20, 7205–7211 (2010a).
Sashidhara, K. V., Rosaiah, J. N., Kumar, M., Gara, R. K., Nayak, L. V., Srivastava, K., Bid, H. K., and Konwar, R., Neo-tanshinlactone inspired synthesis, in vitro evaluation of novel substituted benzocoumarin derivatives as potent anti-breast cancer agents. Bioorg. Med. Chem. Lett., 20, 7127–7131 (2010b).
Sharma, G. N., Dave, R., Sanadya, J., Sharma, P., and Sharma, K. K., Various types and management of breast cancer: an overview. J. Adv. Pharm. Technol. Res., 1, 109–126 (2010).
Sivakumar, P. M., Priya, S., and Doble, M., Synthesis, biological evaluation, mechanism of action and quantitative structure-activity relationship studies of chalcones as antibacterial agents. Chem. Biol. Drug Des., 73, 403–415 (2009).
Zhang, Y., Shi, S., Zhao, M., Jiang, Y., and Tu, P., A novel chalcone from Coreopsis tinctoria nutt. Biochem. Syst. Ecol., 34, 766–769 (2006).
Zhou, Z. Z., Huang, W., Ji, F. Q., Ding, M. W., and Yang, G. F., Construction of a combinatorial library of 2-(4-oxo-4H-1-benzopyran-3-yl)-4-thiazolidinones by microwave-assisted one-pot parallel syntheses. Heteroatom Chem., 18, 381–389 (2007).
Yayli, N., Ucuncu, O., Yasar, A., Gok, Y., Kucuk, M., and Kolayli, S., Stereoselective photochemistry of methoxy chalcones in solution and their radical scavenging activity. Turk. J. Chem., 28, 515–521 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Firoozpour, L., Edraki, N., Nakhjiri, M. et al. Cytotoxic activity evaluation and QSAR study of chromene-based chalcones. Arch. Pharm. Res. 35, 2117–2125 (2012). https://doi.org/10.1007/s12272-012-1208-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-012-1208-2