Abstract
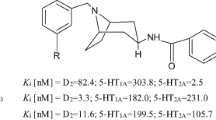
On the basis of our earlier studies, a series of N-{4-[4-(aryl) piperazin-1-yl]-phenyl}-amine derivatives containing terminal carbamoyl fragment with alkyl spacer of different lengths (15–20) were synthesized as ligands, for 5-hydroxytryptamine-1A (5-HT1A) receptor. Molecular modeling studies were undertaken to explain the influence of spacer length on ligands affinity towards 5-HT1A receptor. Compound 19 showed all the specific interactions responsible for recognition. The protonated amine of the ligand forms an ionic hydrogen bond with the negatively charged Asp116 of transmembrane3 helix (TM3), while the carbamoyl moiety interacts with Asn386 and Tyr390 of TM7. The aryl group is involved in forming a CH-π interaction with Phe362. The strong interaction of compound 19 with 5-HT1A receptor in docking studies was confirmed by radio ligand binding studies. Compound 19 showed high affinity for the receptor (Ki = 0.018 nM). In vivo pharmacological testing of compound 19 (3 mg/kg body weight) showed increased open arm entries, as well as time spent in Elevated plus Maze test. Toxicological analysis also revealed no significant biochemical or morphological alterations in the vital organs of experimental animals. Furthermore our results suggest that these compounds share some pharmacological effects with established anxiolytics and might prove to be effective compounds for the treatment of anxiety.
Similar content being viewed by others
References
Alam, A. H. M. K., Islam, R., Salam, K. A., Manir, M. M., Baki, M. A., and Hossain, M. A., Toxicological studies of N-transferloyl-4-methyldopamine isolated from Achranthes ferruginea. Pak. J. Biol. Sci., 9, 1052–1055 (2006).
Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., Weissig, H., Shindyalov, I. N., and Bourne, P. E., The Protein Data Bank. Nucleic Acids Res., 28, 235–242 (2002).
Bergmeyer, H. U. and Bernt, E., In Methods of Enzymatic Analysis, Bergmeyer, H. U. (ed.), Academic Press, New York, pp. 727–733, (1974).
Buhot, M. C., Martin, S., and Segu, L., Role of serotonin in memory impairment. Ann. Med., 32, 210–221 (2000).
Costa-Silva, J. H., Lyra, M. M., Lima, C. R., Arruda, V. M., Araújo, A. V., Ribeiro e Ribeiro, A., Arruda, A. C., Fraga, M. C., Lafayette, S. S., and Wanderley, A. G., A toxicological evaluation of the effect of Carapa guianensis Aublet on pregnancy in Wistar rats. J. Ethnopharmacol., 112, 122–126 (2007).
Dawson, G. R. and Tricklebank, M. D., Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol. Sci., 16, 33–36 (1995).
Garattini, S., Bizzi, A., Caccia, S., Mennini, T., and Samanin, R., Progress in assessing the role of serotonin in the control of food intake. Clin. Neuropharmacol., 11Suppl 1, S8–S32 (1988).
Gozlan, H., Mestikawy, S., Pichat, L., Glowinski, J., and Hamon, M., Identification of presynaptic serotonin autoreceptors using a new ligand: 3H-PAT. Nature, 305, 140–142 (1983).
Hanson, M. A., Cherezov, V., Grifth, M. T., Roth, C. B., Jaakola, V. P., Chien, E. Y. T., Velasquez, J., Kuhn, P., and Stevens, R. C., A specic cholesterol binding site is established by the 2.8 Å structure of the human beta2-adrenergic receptor. Structure, 16, 897–905 (2008).
Hoyer, D., Hannon, J. P., and Martin, G. R., Molecular, pharmacological and functional diversity of 5-H Treceptors. Pharmacol. Biochem. Behav., 71, 533–554 (2002).
Khatri, M., Rai, S. K., Alam, S., Vij, A., and Tiwari, M., Synthesis and pharmacological evaluation of new arylpiperazines N-[4-[4-(aryl) piperazine-1-yl]-phenyl]-amine derivatives: putative role of 5-HT1A receptors. Bioorg. Med. Chem., 17, 1890–1897 (2009).
Kiss, R., Noszál, B., Rácz, A., Falus, A., Eros, D., and Keseru, G. M., Binding mode analysis and enrichment studies on homology models of the human histamine H4 receptor. Eur. J. Med. Chem., 43, 1059–1070 (2008).
Kowalski, P., Jaśkowska, J., Bojarski, A. J., Duszyńska, B., Bucki, A., and Kołaczkowski, M., Evaluation of 1-arylpiperazine derivative of hydroxybenzamides as 5-HT1A and 5-HT7 serotonin receptor ligands: An experimental and molecular modeling approach. J. Heterocycl. Chem., 48, 192–198 (2011).
Latha, R. M., Geentha, T., and Varalakshmi, P., Effect of Vernonia cinerea Less. flower extract in adjuvant-induced arthritis. Gen. Pharmacol., 39, 601–606 (1998).
Liapakis, G., Ballesteros, J. A., Papachristou, S., Chan, W. C., Chen, X., and Javitch, J. A., The forgotten Serine: A critical role for Ser-2035.42 in ligand binding to and activation of the beta 2 adrenergic receptor. J. Biol. Chem., 275, 37779–37788 (2000).
Lopez-Rodriguez, M. L., Ayala, D., Benhamu, B., Morcillo, M. J., and Viso, A., Arylpiperazine derivatives acting at 5-HT1A receptors. Curr. Med. Chem., 9, 443–469 (2002).
Medina, R. A., Sallander, J., Benhamú, B., Porras, E., Campillo, M., Pardo, L., and López-Rodríguez, M. L., Synthesis of New Serotonin 5-HT7 Receptor Ligands. Determinants of 5-HT7/5-HT1A Receptor Selectivity. J. Med. Chem., 52, 2384 (2009).
MOE version 2009.10, Chemical Computing Group: Montreal, Canada. http://www.chemcomp.com
Murakami, M. and Kouyama, T., Crystal structure of squid rhodopsin. Nature, 453, 363–367 (2008).
Obniska, J., Kołaczkowski, M., Bojarski, A. J., and Duszyñska, B., Synthesis, anticonvulsant activity and 5-HT1A, 5-HT2A receptor affinity of new N-[(4-arylpiperazin-1-yl)-alkyl] derivatives of 2-azaspiro[4.4]nonane and [4.5]decane-1,3-dione. Eur. J. Med. Chem., 41, 874–881 (2006).
Okada, T., Sugihara, M., Bondar, A.-N., Elstner, M., Entel, P., and Buss, V., The retinal conformation and its environment in rhodopsin in light of a new 2.2 Å crystal structure. J. Mol. Biol., 342, 571–583 (2004).
Rasmussen, S. G. F., Choi, H. J., Rosenbaum, D. M., Kobilka, T. S., Thian, F. S., Edwards, P. C., Burghammer, M., Ratnala, V. R. P., Sanishvili, R., Fischetti, R. F., Schertler, G. F. X., Weis, W. I., and Kobilka, B. K., Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature, 450, 383–387 (2007).
Raza, M., Al-Shabanath, O. A., El-Hadiyah, T. M., and Al-Majed, A. A., Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci. Pharm., 70, 135–145 (2002).
Schlegel, J. R. and Peroutka, S. J., Nucleotide interactions with 5-HT1A binding sites directly labelled by [3H]-8-hydroxy-2-(di-n-propylamino)tetralin[3H]-8OH-DPAT). Biochem. Pharmacol., 35, 1943–1949 (1986).
The Organisation of Economic Co-operation and Development (OECD), The OECD Guideline for Testing of Chemical: 407 Repeated Dose Oral Toxicity-Rodent: 28-day or 14-day Study, OECD, Paris, pp. 1–7, (2001a).
The Organisation of Economic Co-operation and Development (OECD), The OECD Guideline for Testing of Chemical: 420 Acute Oral Toxicity, OECD, Paris, pp. 1–14, (2001b).
Walf, A. A. and Frye, C. A., The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc., 2, 322–328 (2007).
Warne, T., Serrano-Vega, M. J., Baker, J. G., Moukhametzianov, R., Edwards, P. C., Henderson, R., Leslie, A. G. W., Tate, C. G., and Schertler, G. F. X., Structure of a beta1-adrenergic G-protein-coupled receptor. Nature, 454, 486–491 (2008).
Waynforth, B. H., Injection Techniques: Experimental and Surgical Techniques in the Rat, Academic Press, London, pp. 3–61, (1980).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khatri, M., Rai, S.K., Ranbhor, R. et al. Synthesis and pharmacological evaluation of [(4-arylpiperazin-1-yl)-alkyl]-carbamic acid ethyl ester derivatives as potential anxiolytic agents. Arch. Pharm. Res. 35, 1143–1152 (2012). https://doi.org/10.1007/s12272-012-0704-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-012-0704-8