Abstract
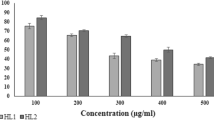
Bromination of visnagin (1) afforded 9-bromovisnagin (2) which on its alkaline hydrolysis afforded the 3-acetyl benzofuran derivative (3). The condensation of (3) with hydrazine hydrate, phenylhydrazine and/or hydroxylamine hydrochloride afforded the corresponding pyrazole derivatives (4a, b) and isoxazole derivative (4c). On the other hand, when compound 3 was condensed with some aromatic aldehydes, this yielded corresponding α, β-unsaturated keto derivatives (5a–e). Furthermore, when 1 was subjected to chlorosulfonation, the visnaginsulfonylchloride derivative 6 was afforded, which on amidation using morpholine, a sulonamido derivative (7) was obtained. Alkaline hydrolysis of the latter compound yielded 7-N-morpholinosulsamidobenzofuran (8) which was condensed with some aromatic aldehydes to yield the corresponding chalcone compounds (9a–e). Demethylation of visnagin afforded norvisnagin (10). The reaction of 10 with ethylbromoacetate in dry acetone yielded the ester benzopyran derivative (11) which reacted with hydrazine hydrate to afford the corresponding hydrazide derivative (12) and this was condensed with 3,4,5-trimethoxybenzaldehyde to give the corresponding hydrazone (13). A thaizolidinone derivative (14) was obtained by condensation of (13) with thioglycolic acid. Chloromethylation of norvisnagin afforded a 4-chloromethyl derivative (15) which reacted with different primary and secondary amines to yield the corresponding ethylamino derivative (16a, b). Moreover, mannich bases (16a, b) and (17a–c) were obtained by reacting norvisnagin with different primary and secondary amines in the presence of formalin but benzoylation of (16a, b) and (17a–c) afforded 4-oxybenzoyl derivative (18a–e). The prepared compounds were tested for their interaction with DNA; bromovisnagin 2 showed the highest affinity and compounds 6, 15, 8a, > 14, > 16b, 17a, and 16a showed moderate activity in decreasing potency. Moreover, compound 2 also was the most active as antiviral agent toward HS-I virus and compounds 6, 7, 15, 14, 16a, and 18a were found to be moderately active. CD50 of the active compounds were also measured.
Similar content being viewed by others
References
Abdel-Alim, M. A. and Aboulezz, A. F., Synthesis of some new furochromones. Egypt J. Chem., 29, 465–470 (1986).
Abd Elhafez, O. M., El Khrisy Eel, D., Badria, F., and Fathy Ael, D., Synthesis and biological investigation of new thiazolidinone and oxadiazoline coumarin derivatives. Arch. Pharm. Res., 26, 686–696 (2003).
Balzarini, J., Orzeszko, B., Maurin, J. K., and Orzeszko, A., Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem., 42, 993–1003 (2007).
Bouabdallah, I., M’Barek, L. A., Zyad, A., Ramdani, A., Zidane, I., and Melhaoui, A., Anticancer effect of three pyrazole derivatives. Nat. Prod. Res., 20, 1024–1030 (2006).
Dimmock, J. R., Elias, D. W., Beazely, M. A., and Kandepu, N. M., Bioactivities of chalcones. Curr. Med. Chem., 6, 1125–1149 (1999).
Duarte, J., Lugnier, C., Torres, A. I., Pérez-Vizcaino, F., Zarzuelo, A., and Tamargo, J., Effects of visnagin on cyclic nucleotide phosphodiesterases and their role in its inhibitory effects on vascular smooth muscle contraction. Gen. Pharmacol., 32, 71–74 (1999).
Duarte, J., Torres, A. I., and Zarzuelo, A., Cardiovascular effects of visnagin on rats. Planta Med., 66, 35–39 (2000).
El-Bendary, E. r. and Badria, F. A., Synthesis, DNA-binding, and antiviral activity of certain pyrazolo[3,4-d]pyrimidine derivatives. Arch. Pharm. (Weinheim), 333, 99–103 (2000).
El-Gamal, M. H. A., Shalaby, N. M. M., Duddeck, H., and Rosenbaum, D., Synthesis of some furochromonesulfonamide derivatives with potential pharmacological activity. J. Heterocycl. Chem., 24, 721–724 (1987).
El-Sherbeny, M. A., El-Ashmawy, M. B., El-Subbagh, H. I., El-Emam, A. A., and Badria, F. A., Synthesis, antimicrobial and antiviral evaluation of certain thienopyrimidine derivatives. Eur. J. Med. Chem., 30, 445–449 (1995).
El-Subbagh, H. I., Abu-Zaid, S. M., Mahran, M. A., Badria, F. A., and Al-Obaid, A. M., Synthesis and biological evaluation of certain alpha,beta-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J. Med. Chem., 43, 2915–2921 (2000).
Galal, S. A., Abd El-All, A. S., Abdallah, M. M., and El-Diwani, H. I., Synthesis of potent antitumor and antiviral benzofuran derivatives. Bioorg. Med. Chem. Lett., 19, 2420–2428 (2009).
García-Giménez, J. L., González-Alvarez, M., Liu-González, M., Macías, B., Borrás, J., and Alzuet, G., Toward the development of metal-based synthetic nucleases: DNA binding and oxidative DNA cleavage of a mixed copper(II) complex with N-(9H-purin-6-yl)benzenesulfonamide and 1,10-phenantroline. Antitumor activity in human Caco-2 cells and Jurkat T lymphocytes. Evaluation of p53 and Bcl-2 proteins in the apoptotic mechanism. J. Inorg. Biochem., 103, 923–934 (2009).
Hishmat, O. H., Mustafa, A., and Younes, M. M. Y., Mannich reaction of 1(4,6-dimethoxybenzofuranyl) and 1-(4,6-7-trimethoxybenzofuranyl) butan-1,2,3-trione-2-aryl hydrazone. Ind. J. Chem., 9, 893–894 (1971).
Kamal, A., Shankaraiah, N., Prabhakar, S., Reddy, Ch. R., Markandeya, N., Reddy, K. L., and Devaiah, V., Solid-phase synthesis of new pyrrolobenzodiazepine-chalcone conjugates: DNA-binding affinity and anticancer activity. Bioorg. Med. Chem. Lett., 18, 2434–2439 (2008).
Kochetkov, N. K. and Sokolov, S. D., In Katritzky, A. R. (Ed.), Advances in Heterocyclic Chemistry. Academic press, New York, Vol. 2, pp. 365–422, (1963).
Lee, J. K., Jung, J. S., Park, S. H., Park, S. H., Sim, Y, B., Kim, S. M., Ha, T. S., and Suh, H. W., Anti-inflammatory effect of visnagin in lipopolysaccharide-stimulated BV-2 microglial cells. Arch. Pharm. Res., 33, 1843–1850 (2010).
Lv, P. C., Zhou, C. F., Chen, J., Liu, P. G., Wang, K. R., Mao, W. J., Li, H. Q., Yang, Y., Xiong, J., and Zhu, H. L., Design, synthesis and biological evaluation of thiazolidinone derivatives as potential EGFR and HER-2 kinase inhibitors. Bioorg. Med. Chem., 18, 314–319 (2010).
Mandour, A. H., Abu-Mustafa, E. A., Abdel-Latif, N. A., and El-Bazza, Z. E., Synthesis and biological evaluation of some new visnagin and benzofuran derivatives. Al-Azhar Bull. Sci., 5, 983–993 (1994).
Mijatovic, T., De Nève, N., Gailly, P., Mathieu, V., Haibe-Kains, B., Bontempi, G., Lapeira, J., Decaestecker, C., Facchini, V., and Kiss, R., Nucleolus and c-Myc: potential targets of cardenolide-mediated antitumor activity. Mol. Cancer Ther., 7, 1285–1296 (2008).
Park, H. J., Lee, K., Park, S. J., Ahn, B., Lee, J. C., Cho, H., and Lee, K. I., Identification of antitumor activity of pyrazole oxime ethers. Bioorg. Med. Chem. Lett., 15, 3307–3312 (2005).
Ragab, F. A., Hassan, G. S., Yossef, H. A., and Hashem, H. A., Synthesis of 6- and 9-alkylaminomethyl furoflavones as gastroprotective agents. Eur. J. Med. Chem., 42, 1117–1127 (2007).
Rao, A., Balzarini, J., Carbone, A., Chimirri, A., De Clercq, E., Monforte, A. M., Monforte, P., Pannecouque, C., and Zappalà, M., 2-(2,6-Dihalophenyl)-3-(pyrimidin-2-yl)-1,3-thiazolidin-4-ones as non-nucleoside HIV-1 reverse transcriptase inhibitors. Antiviral Res., 63, 79–84 (2004).
Rescifina, A., Chiacchio, M. A., Corsaro, A., De Clercq, E., Iannazzo, D., Mastino, A., Piperno, A., Romeo, G., Romeo, R., and Valveri, V., Synthesis and biological activity of isoxazolidinyl polycyclic aromatic hydrocarbons: potential DNA intercalators. J. Med. Chem., 49, 709–715 (2006).
Rida, S. M., El-Hawash, S. A., Fahmy, H. T., Hazzaa, A. A., and El-Meligy, M. M., Synthesis of novel benzofuran and related benzimidazole derivatives for evaluation of in vitro anti-HIV-1, anticancer and antimicrobial activities. Arch. Pharm. Res., 29, 826–833 (2006).
Schönberg, A. and Aziz, G., Furochromones and coumarins. VI. Demethylation of xanthotoxin, khellin and khellol with aniline hydrochloride and magnesium iodide. J. Am. Chem. Soc., 75, 3265–3266 (1953).
Scozzafava, A., Owa, T., Mastrolorenzo, A., and Supuran, C. T., Anticancer and antiviral sulfonamides. Curr. Med. Chem., 10, 925–953 (2003).
Shivarama Holla, B., Veerendra, B., Shivananda, M. K., and Poojary, B., Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur. J. Med. Chem., 38, 759–767 (2003).
Sriram, D., Yogeeswari, P., and Kumar, T. G., Microwaveassisted synthesis and anti-YFV activity of 2,3-diaryl-1,3-thiazolidin-4-ones. J. Pharm. Pharm. Sci., 8, 426–429 (2005).
Starkowsky, N. A., Addition of urea, thiourea and iodine to the natural benzopyrones of Ammi visnaga Linn. and Ammi majus linn. Egypt J. Chem., 2, 111–117 (1959).
Tan, J. H., Zhang, Q. X., Huang, Z. S., Chen, Y., Wang, X. D., Gu, L. Q., and Wu, J. Y., Synthesis, DNA binding and cytotoxicity of new pyrazole emodin derivatives. Eur. J. Med. Chem., 41, 1041–1047 (2006).
Terzioğlu, N., Karali, N., Gürsoy, A., Pannecouque, C., Leysen, P., Paeshuyse, J., Neyts, J., and De Clercq, E., Synthesis and primary antiviral activity evaluation of 3-hydrazono-5-nitro-2-indolinone derivatives. ARKIVOC, (i), 109–118 (2006).
Wenzel, N. I., Chavain, N., Wang, Y., Friebolin, W., Maes, L., Pradines, B., Lanzer, M., Yardley, V., Brun, R., Herold-Mende, C., Biot, C., Tóth, K., and Davioud-Charvet, E., Antimalarial versus cytotoxic properties of dual drugs derived from 4-aminoquinolines and Mannich bases: interaction with DNA. J. Med. Chem., 53, 3214–3226 (2010).
Wu, S., Guo, W., Teraishi, F., Pang, J., Kaluarachchi, K., Zhang, L., Davis, J., Dong, F., Yan, B., and Fang, B., Anticancer activity of 5-benzylidene-2-phenylimino-1,3-thiazolidin-4-one (BPT) analogs. Med. Chem., 2, 597–605 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdelhafez, O.M., Abedelatif, N.A. & Badria, F.A. DNA binding, antiviral activities and cytotoxicity of new furochromone and benzofuran derivatives. Arch. Pharm. Res. 34, 1623–1632 (2011). https://doi.org/10.1007/s12272-011-1006-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-011-1006-2