Abstract
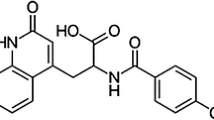
The polymorphic and pseudopolymorphic forms of CG-400549, a novel FabI inhibitor with potent in vivo activity were prepared and characterized by differential scanning calorimetry (DSC), powder X-ray diffractometry (PXRD) and thermogravimetric analysis (TG). Seven crystal forms of CG-400549, one anhydrate and six solvates, have been isolated by recrystallization and the DSC and PXRD patterns of the seven crystal forms of CG-400549 were different respectively. The dissolution patterns of these seven crystal forms of CG-400549 were studied and they showed significant differences in the dissolution rate. After storage of 1 month at 0% RH (silica gel, 20°C), 52% RH (saturated solution of Na2Cr2O72H2O/20°C) and 95% RH (saturated solution of Na2HPO4/20°C), all crystal forms were not transformed.
Similar content being viewed by others
References
Brittain, H. G., Polymorphism in Pharmaceutical Solids. Marcel Dekker, New York. Basel, pp. 246, (1999).
Campeta, A. M., Chekal, B. P., Abramov, Y. A., Meenan, P. A., Henson, M. J., Shi, B., Singer, R. A., and Horspool, K. R., Development of a targeted polymorph screening approach for a complex polymorphic and highly solvating API. J. Pharm. Sci., 99, 3874–3886 (2010).
Ceolin, R., Toscani, S., Gardette, M. F., Agafonov, V. N., Dzyabchenko, A. V., and Bachet, B., X-ray characterization of the triclinic polymorph of carbamazepine. J. Pharm. Sci., 86, 1062–1065 (1997).
Giron, D., Thermal analysis and calorimetric methods in the characterization of polymorphs and solvates. Thermochim. Acta, 248, 1–59 (1995).
Haleblian, J. K. and McCrone, W. C., Pharmaceutical applications of polymorphism. J. Pharm. Sci., 58, 911–929 (1969).
Haleblian, J. K., Characterization of habits and crystalline modification of solids and their pharmaceutical applications. J. Pharm. Sci., 64, 1269–1288 (1975).
Hüttenrauch, R., Fundamentals of pharmaceutics. Acta Pharm. Technol., 34, 1–10 (1988).
Kobayashi, Y., Ito, S., Itai, S., and Yamamoto, K., Physicochemical properties and bioavailability of carbamazepine polymorphs and dihydrate. Int. J. Pharm., 193, 137–146 (2000).
Kuhnert-Brandstätter, M. and Burger, A., Untersuchungen zum Aufldesungsverhalten polymorpher, pseudopolymorpher und amorpher Phasen von Arzneimitteln. Pharm. Ind., 34, 187–190 (1972).
Kuhnert-Brandst↦ter, M. and Lehner, G., Differentialthermoanalytische und IR-spektroskopische Untersuchungen von Arzneistoffen, die als Hydrate vorliegen. Sci. Pharm., 52, 267–279 (1984).
Lachman, L., Lieberman, H. A., and Kanig, J. L., The Theory and Practice of Industrial Pharmacy. Lea & Febiger, Philadelphia, pp. 171, (1986).
Lee, E.-A. and Sohn, Y.-T., Crystal forms of a capsaicin derivative analgesic DA-5018. J. Therm. Anal. Calorim., 93, 871–874 (2008).
Opalchenova, G. and Kalinkova, G. N., Evaluation of a new polymorph azlocillin sodium by its antibacterial activity. Int. J. Pharm., 153, 263–265 (1997).
Poole, J. W. and Bahal, C. K., Dissolution behavior and solubility of anhydrous and trihydrate forms of ampicillin. J. Pharm. Sci., 57, 1945–1948 (1968).
Sohn, Y. T., Crystal forms of an angiotensin II receptor antagonist BR-A657. J. Therm. Anal. Calorim., 89, 799–802 (2007).
Song, H. O. and Sohn, Y. T., Crystal forms of SK-3530. Arch. Pharm. Res., 33, 2033–2036 (2010).
Yamamura, S. and Momose, Y., Quantitative analysis of crystalline pharmaceuticals in powders and tablets by a pattern-fitting procedure using X-ray powder diffraction data. Int. J. Pharm., 212, 203–212 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, BY., Sohn, YT. Solid state of CG-400549, a novel FabI inhibitor: Characterization, dissolution, transformation. Arch. Pharm. Res. 34, 775–779 (2011). https://doi.org/10.1007/s12272-011-0511-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-011-0511-7