Abstract
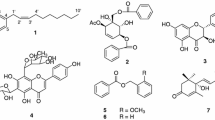
Five flavonoids, myricetin-3′-methylether 3-O-β-d-galactopyranoside (1), myricetin-3′,5′-dimethylether 3-O-β-d-galactopyranoside (2), quercetin (3), kaempferol (4), and tamarixetin (5) were isolated from the buds of Cleistocalyx operculatus (Myrtaceae). The chemical structures of these compounds were determined on the basis of spectroscopic analyses, including 2D NMR. Their anti-Alzheimer effects were evaluated via acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activity assays. All five compounds 1–5 showed potential inhibitory activities against AChE with IC50 values of 19.9, 37.8, 25.9, 30.4 and 22.3 μM, respectively, while compounds 1, 3, 4 and 5 also possessed BChE inhibitory activity with IC50 values of 152.5, 177.8, 62.5, and 160.6 μM, respectively.
Similar content being viewed by others
References
Braca, A., Bilia, A. R., Mendez, J., and Morelli, I., Myricetin glycosides from Licania densiflora. Fitoterapia, 72, 182–185 (2001).
Dung, N. T., Bajpai, V. K., Yoon, J. I., and Kang, S. C., Antiinflammatory effects of essential oil isolated from the buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Food Chem. Toxicol., 47, 449–453 (2009).
Ellman, G. L., Courtney, K. D., Andres, V. Jr., and Featherstone, R. M., A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol., 7, 88–95 (1961).
Fan, P., Hay, A. E., Marston, A., and Hostettmann, K., Acetylcholinesterase-inhibitory activity of linarin from Buddleja davidii, structure-activity relationships of related flavonoids, and chemical investigation of Buddleja nitida. Pharm. Biol., 46, 596–601 (2008).
Harborne, J. B., Correlations between flavonoid chemistry, anatomy and geography in the restionaceae. Phytochemistry, 18, 1323–1327 (1979).
Ji, H. F. and Zhang, H. Y., Theoretical evaluation of flavonoids as multipotent agents to combat Alzheimer’s disease. J. Mol. Struct., 767, 3–9 (2006).
Lim, S. S., Han, S. M., Kim, S. Y., Bae, Y. S., and Kang, I. J., Isolation of acetylcholinesterase inhibitors from the flowers of Chrysanthemum indicum Linne. Food Sci. Biotechnol., 16, 265–269 (2007).
Lu, Y. H., Du, C. B., Wu, Z. B., Ye, C. L., Liu, J. W., and Wei, D. Z., Protective effects of Cleistocalyx operculatus on lipid peroxidation and trauma of neuronal cells. Zhongguo Zhong Yao Za Zhi, 28, 964–966 (2003).
Nomura, M., Yamakawa, K., Hirata, Y., and Niwa, M., Antidermatophytic constituent from the bark of Cleistocalyx operculatus. Shoyakugaku Zasshi, 47, 408–410 (1993).
Parihar, M. S. and Hemnani, T., Alzheimer’s disease pathogenesis and therapeutic interventions. J. Clin. Neurosci., 11, 456–467 (2004).
Rao, A. A., Sridhar, G. R., and Das, U. N., Elevated butyrylcholinesterase and acetylcholinesterase may predict the development of type 2 diabetes mellitus and Alzheimer’s disease. Med. Hypotheses, 69, 1272–1276 (2007).
Sawasdee, P., Sabphon, C., Sitthiwongwanit, D., and Kokpol, U., Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res., 23, 1792–1794 (2009).
Scarpini, E., Scheltens, P., and Feldman, H., Treatment of Alzheimer’s disease: current status and new perspectives. Lancet Neurol., 2, 539–547 (2003).
Thuong, P. T., Kang, H. J., Na, M., Jin, W., Youn, U. J., Seong, Y. H., Song, K. S., Min, B. S., and Bae, K., Anti-oxidant constituents from Sedum takesimense. Phytochemistry, 68, 2432–2438 (2007).
Vassar, R., Beta-secretase (BACE) as a drug target for Alzheimer’s disease. Adv. Drug Deliv. Rev., 54, 1589–1602 (2002).
Woo, A. Y., Waye, M. M., Kwan, H. S., Chan, M. C., Chau, C. F., and Cheng, C. H., Inhibition of ATPases by Cleistocalyx operculatus. A possible mechanism for the cardiotonic actions of the herb. Vascul. Pharmacol., 38, 163–168 (2002).
Yan, R., Bienkowski, M. J., Shuck, M. E., Miao, H., Tory, M. C., Pauley, A. M., Brashier, J. R., Stratman, N. C., Mathews, W. R., Buhl, A. E., Carter, D. B., Tomasselli, A. G., Parodi, L. A., Heinrikson, R. L., and Gurney, M. E., Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature, 402, 533–537 (1999).
Ye, C. L., Lu, Y. H., and Wei, D. Z., Flavonoids from Cleistocalyx operculatus. Phytochemistry, 65, 445–447 (2004).
Ye, C. L., Liu, J. W., Wei, D. Z., Lu, Y. H., and Qian, F., In vivo antitumor activity by 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone in a solid human carcinoma xeno-graft model. Cancer Chemother. Pharmacol., 56, 70–74 (2005a).
Ye, C. L., Liu, Y., and Wei, D. Z., Antioxidant and anticancer activity of 3′-formyl-4′,6′-dihydroxy-2′-methoxy-5′-methylchalcone and (2S)-8-formyl-5-hydroxy-7-methoxy-6-methylflavanone. J. Pharm. Pharmacol., 59, 553–559 (2007).
Ye, C. L., Qian, F., Wei, D. Z., Lu, Y. H., and Liu, J. W., Induction of apoptosis in K562 human leukemia cells by 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone. Leuk. Res., 29, 887–892 (2005b).
Zhang, F. X., Liu, M. F., and Lu, R. R., Chemical constituents from the buds of Cleistocalyx operculatus. Zhiwu Xuebao, 32, 469–472 (1990).
Zhang, H. Y., Yang, D. P., and Tang, G. Y., Multipotent antioxidants: from screening to design. Drug Discov. Today, 11, 749–754 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Min, B.S., Cuong, T.D., Lee, JS. et al. Cholinesterase inhibitors from Cleistocalyx operculatus buds. Arch. Pharm. Res. 33, 1665–1670 (2010). https://doi.org/10.1007/s12272-010-1016-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-010-1016-5