Abstract
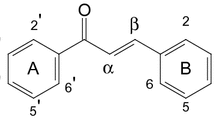
A new isoflavone glycoside, 6-methoxy-7-hydroxy-4′-O-β-d-glucosyl isoflavone, glycitein-4′-O-β-d-glucoside (10), along with nine known flavonoids, were isolated from the stem bark of Sophora japonica. The structures of these compounds were determined by analysis of spectroscopic data (1D -, 2D - NMR and HRMS). The inhibitory effects of all the isolated compounds on aldose reductase were evaluated in vitro. Among these compounds, daidzein (1), puerol A (4), and paratensein-7-O-glucoside (9) exhibited potent inhibitory effects, with IC50 values of 3.2, 6.4, and 1.9 μM, respectively.
Similar content being viewed by others
References
Anut, S., Zinsmeister, H. D., Mues, R., Barz, W., Mackenbrock, K., Kôster, J., and Markham, R. K., The first identification of iosflavones from a Bryophyte. Phytochemistry, 23, 1073–1075 (1984).
Editorial Committee of Zhong Hua Ben Cao of State Administration of Traditional Chinese Medicine of People’s Republic of China. Zhong Hua Ben Cao, Vol. 4. Shanghai Science Technology Publishing Company, Shanghai, pp. 3389, (1999).
Feldman, E. L., Stevens, M. J., and Greene, D. A., Pathogenesis of diabetic neuropathy. Clin. Neurosci., 4, 365–370 (1997).
Hayman, S. and Kinoshita, J. H., Isolation and properties of lens aldose reductase. J. Biol. Chem., 240, 877–882 (1965).
Hwang, M. H., Kwon, Y. S., and Kim, C. M., Isoflavone compounds of the heartwood of Maackia fauriei. Yakhakhoe Chi, 41, 444–449 (1997).
Laman, N. A. and Volynets, A. P., Isoflavones of the roots of Lupinus luteus. Chem. Nat. Comp., 10, 175–177 (1975).
Lee, E. J., Yean, M. H., Jung, H. S., Kim, J. S., and Kang, S. S., Phytochemical studies on Astragalus root (2) - Flavonoids and a Lignan. Nat. Prod. Sci., 14, 131–137 (2008).
Naim, M., Gestetner, B., Zilkah, S., Birk, Y., and Bondi, A., Soybean isoflavones. Characterization, determination, and antifungal activity. J. Agric. Food Chem., 22, 806–810 (1974).
Nishimura, C., Yamaoka, T., Mizutani, M., Yamasita, K., Akera, T., and Tanimoto, T., Purification and characterization of the recombinant human aldose reductase espressed in baculovirus system. Biochim. Biophys. Acta, 1078, 171–181 (1991).
Park, H. J., Park, J. H., Moon, J. O., Lee, K. T., Jung, W. T., Oh, S. R., and Lee, H. K., Isoflavone glycosides from the flowers of Pueraria thunbergiana. Phytochemistry, 51, 147–151 (1999).
Park, Y. K., Lee, H. J., Choi, D. H., Kwon, Y. H., and Oh, J. S., Extractives from the Bark of Sophora japonica L. Mokchae Konghak, 30, 42–47 (2002).
Santiago, J. V., Lessons from the diabetes control and complications trial. Diabetes, 42, 1549–1554 (1993).
Yabe-Nishimura, C., Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacol. Rev., 50, 21–33 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Park, H.Y., Kim, S.H., Kim, G.B. et al. A new isoflavone glycoside from the stem bark of Sophora japonica . Arch. Pharm. Res. 33, 1165–1168 (2010). https://doi.org/10.1007/s12272-010-0805-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-010-0805-1