Abstract
Plasma ceramides (Cer), a subset of bioactive lipids, have mechanistic links to development of atherosclerosis and are related to major adverse cardiovascular events (MACEs). Previous researches have demonstrated vulnerable plaques contribute to acute cardiovascular events and poor prognosis. This study aimed to explore the associations between Cer and culprit plaque characterizations evaluated by optical coherence tomography (OCT). It was found that plasma Cer are associated with culprit plaque vulnerability evaluated by OCT, providing evidence supporting proatherogenic roles and potential to act as markers for plaque vulnerability of Cer.

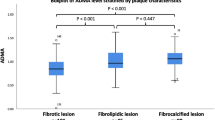
With increasing plasma ceramide levels, the prevalence of thin-cap fibroatheroma (TCFA) and plaque rupture (PR) is higher, that is, culprit plaques are more vulnerable.




Similar content being viewed by others
Abbreviations
- Cer:
-
Ceramide
- CHD:
-
Coronary heart disease
- STEMI:
-
St-segment elevation myocardial infarction
- OCT:
-
Optical coherence tomography
- PR:
-
Plaque rupture
- LRP:
-
Lipid-rich plaque
- TCFA:
-
Thin-cap fibroatheroma
- FCT:
-
Fibrous cap thickness
- RRLC-Q-TOF/MS:
-
Rapid resolution liquid chromatography coupled with quadrupole time-of-flight mass spectrometry
- MACEs:
-
Major adverse cardiovascular events
- Hs-CRP:
-
High-sensitive c-reactive protein
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
References
Stith, J. L., Velazquez, F. N., & Obeid, L. M. (2019). Advances in determining signaling mechanisms of ceramide and role in disease. Journal of Lipid Research, 60(5), 913–918. https://doi.org/10.1194/jlr.S092874.
Edsfeldt, A., Duner, P., Stahlman, M., Mollet, I. G., Asciutto, G., Grufman, H., et al. (2016). Sphingolipids contribute to human atherosclerotic plaque inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 36(6), 1132–1140. https://doi.org/10.1161/ATVBAHA.116.305675.
Levade, T., Augé, N., Veldman, R. J., Cuvillier, O., Nègre-Salvayre, A., & Salvayre, R. (2001). Sphingolipid mediators in cardiovascular cell biology and pathology. Circulation Research, 89(11), 957–968. https://doi.org/10.1161/hh2301.100350.
Xu, X., Gao, B., Guan, Q., Zhang, D., Ye, X., Zhou, L., et al. (2016). Metabolomic profile for the early detection of coronary artery disease by using UPLC-QTOF/MS. Journal of Pharmaceutical and Biomedical Analysis, 129, 34–42. https://doi.org/10.1016/j.jpba.2016.06.040.
Lallemand, T., Rouahi, M., Swiader, A., Grazide, M. H., Geoffre, N., Alayrac, P., et al. (2018). nSMase2 (type 2-neutral sphingomyelinase) deficiency or inhibition by GW4869 reduces inflammation and atherosclerosis in Apoe(-/-) mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 38(7), 1479–1492. https://doi.org/10.1161/ATVBAHA.118.311208.
Park, T. S., Rosebury, W., Kindt, E. K., Kowala, M. C., & Panek, R. L. (2008). Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacological Research, 58(1), 45–51. https://doi.org/10.1016/j.phrs.2008.06.005.
Wang, D. D., Toledo, E., Hruby, A., Rosner, B. A., Willett, W. C., Sun, Q., et al. (2017). Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (Prevencion con Dieta Mediterranea). Circulation, 135(21), 2028–2040. https://doi.org/10.1161/CIRCULATIONAHA.116.024261.
de Carvalho, L. P., Tan, S. H., Ow, G. S., Tang, Z., Ching, J., Kovalik, J. P., et al. (2018). Plasma ceramides as prognostic biomarkers and their arterial and myocardial tissue correlates in acute myocardial infarction. JACC Basic Translational Science, 3(2), 163–175. https://doi.org/10.1016/j.jacbts.2017.12.005.
Laaksonen, R., Ekroos, K., Sysi-Aho, M., Hilvo, M., Vihervaara, T., Kauhanen, D., et al. (2016). Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. European Heart Journal, 37(25), 1967–1976. https://doi.org/10.1093/eurheartj/ehw148.
Meeusen, J. W., Donato, L. J., Bryant, S. C., Baudhuin, L. M., Berger, P. B., & Jaffe, A. S. (2018). Plasma ceramides. Arteriosclerosis Thrombosis and Vascular Biology, 38(8), 1933–1939. https://doi.org/10.1161/ATVBAHA.118.311199.
Ali, Z. A., Karimi Galougahi, K., Maehara, A., Shlofmitz, R. A., Ben-Yehuda, O., Mintz, G. S., et al. (2017). Intracoronary optical coherence tomography 2018: current status and future directions. JACC. Cardiovascular Interventions, 10(24), 2473–2487. https://doi.org/10.1016/j.jcin.2017.09.042.
Burgmaier, M., Milzi, A., Dettori, R., Burgmaier, K., Marx, N., & Reith, S. (2018). Co-localization of plaque macrophages with calcification is associated with a more vulnerable plaque phenotype and a greater calcification burden in coronary target segments as determined by OCT. PLoS One, 13(10), e0205984. https://doi.org/10.1371/journal.pone.0205984.
Sinclair, H., Bourantas, C., Bagnall, A., Mintz, G. S., & Kunadian, V. (2015). OCT for the identification of vulnerable plaque in acute coronary syndrome. JACC: Cardiovascular Imaging, 8(2), 198–209. https://doi.org/10.1016/j.jcmg.2014.12.005.
Ibanez, B., James, S., Agewall, S., Antunes, M. J., Bucciarelli-Ducci, C., Bueno, H., et al. (2018). 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal, 39(2), 119–177. https://doi.org/10.1093/eurheartj/ehx393.
Jia, H., Abtahian, F., Aguirre, A. D., Lee, S., Chia, S., Lowe, H., et al. (2013). In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. Journal of the American College of Cardiology, 62(19), 1748–1758. https://doi.org/10.1016/j.jacc.2013.05.071.
Monnin, C., Ramrup, P., Daigle-Young, C., & Vuckovic, D. (2018). Improving negative liquid chromatography/electrospray ionization mass spectrometry lipidomic analysis of human plasma using acetic acid as a mobile-phase additive. Rapid Communications in Mass Spectrometry, 32(3), 201–211. https://doi.org/10.1002/rcm.8024.
Sarafian, M. H., Gaudin, M., Lewis, M. R., Martin, F. P., Holmes, E., Nicholson, J. K., et al. (2014). Objective set of criteria for optimization of sample preparation procedures for ultra-high throughput untargeted blood plasma lipid profiling by ultra performance liquid chromatography-mass spectrometry. Analytical Chemistry, 86(12), 5766–5774. https://doi.org/10.1021/ac500317c.
Bismuth, J., Lin, P., Yao, Q., & Chen, C. (2008). Ceramide: a common pathway for atherosclerosis? Atherosclerosis, 196(2), 497–504. https://doi.org/10.1016/j.atherosclerosis.2007.09.018.
Freed, J. K., Beyer, A. M., LoGiudice, J. A., Hockenberry, J. C., & Gutterman, D. D. (2014). Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circulation Research, 115(5), 525–532. https://doi.org/10.1161/circresaha.115.303881.
Schissel, S. L., Tweedie-Hardman, J., Rapp, J. H., Graham, G., Williams, K. J., & Tabas, I. (1996). Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. The Journal of Clinical Investigation, 98(6), 1455–1464. https://doi.org/10.1172/JCI118934.
Brown, B. A., Williams, H., & George, S. J. (2017). Evidence for the involvement of matrix-degrading metalloproteinases (MMPs) in atherosclerosis. Progress in Molecular Biology and Translational Science, 147, 197–237. https://doi.org/10.1016/bs.pmbts.2017.01.004.
Thim, T., Hagensen, M. K., Bentzon, J. F., & Falk, E. (2008). From vulnerable plaque to atherothrombosis. Journal of Internal Medicine, 263(5), 506–516. https://doi.org/10.1111/j.1365-2796.2008.01947.x.
Falk, E. (2006). Pathogenesis of atherosclerosis. Journal of the American College of Cardiology, 47(8 Suppl), C7–C12. https://doi.org/10.1016/j.jacc.2005.09.068.
Li, H., Junk, P., Huwiler, A., Burkhardt, C., Wallerath, T., Pfeilschifter, J., et al. (2002). Dual effect of ceramide on human endothelial cells. Circulation, 106(17), 2250–2256. https://doi.org/10.1161/01.cir.0000035650.05921.50.
Reith, S., Milzi, A., Lemma, E. D., Dettori, R., Burgmaier, K., Marx, N., et al. (2019). Intrinsic calcification angle: a novel feature of the vulnerable coronary plaque in patients with type 2 diabetes: an optical coherence tomography study. Cardiovascular Diabetology, 18(1), 122. https://doi.org/10.1186/s12933-019-0926-x.
Milzi, A., Burgmaier, M., Burgmaier, K., Hellmich, M., Marx, N., & Reith, S. (2017). Type 2 diabetes mellitus is associated with a lower fibrous cap thickness but has no impact on calcification morphology: an intracoronary optical coherence tomography study. Cardiovascular Diabetology, 16(1), 152. https://doi.org/10.1186/s12933-017-0635-2.
Fretts, A. M., Jensen, P. N., Hoofnagle, A., McKnight, B., Howard, B. V., Umans, J., et al. (2019). Plasma ceramide species are associated with diabetes risk in participants of the strong heart study. The Journal of Nutrition. https://doi.org/10.1093/jn/nxz259.
Raichur, S., Brunner, B., Bielohuby, M., Hansen, G., Pfenninger, A., Wang, B., et al. (2019). The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol Metab, 21, 36–50. https://doi.org/10.1016/j.molmet.2018.12.008.
Huynh, K. (2016). Coronary artery disease: ceramides predict CV death in stable CAD and ACS. Nature Reviews Cardiology, 13(7), 381. https://doi.org/10.1038/nrcardio.2016.81.
Janis, M. T., Tarasov, K., Ta, H. X., Suoniemi, M., Ekroos, K., Hurme, R., et al. (2013). Beyond LDL-C lowering: distinct molecular sphingolipids are good indicators of proprotein convertase subtilisin/kexin type 9 (PCSK9) deficiency. Atherosclerosis, 228(2), 380–385. https://doi.org/10.1016/j.atherosclerosis.2013.03.029.
Ng, T. W., Ooi, E. M., Watts, G. F., Chan, D. C., Weir, J. M., Meikle, P. J., et al. (2014). Dose-dependent effects of rosuvastatin on the plasma sphingolipidome and phospholipidome in the metabolic syndrome. The Journal of Clinical Endocrinology and Metabolism, 99(11), E2335–E2340. https://doi.org/10.1210/jc.2014-1665.
Tarasov, K., Ekroos, K., Suoniemi, M., Kauhanen, D., Sylvanne, T., Hurme, R., et al. (2014). Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. The Journal of Clinical Endocrinology and Metabolism, 99(1), E45–E52. https://doi.org/10.1210/jc.2013-2559.
Funding
This study was supported by research grants from the National Key R&D Program of China (2016YFC1301100) to Dr. Yu, grants from the Laboratory of Myocardial Ischemia, Harbin Medical University, Chinese Ministry of Education (KF201807) to Dr. Pan and grants from National Natural Science Foundation of China (81901853) to Dr. Yang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human Subjects/Informed Consent Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Additional information
Associate Editor Craig M. Stolen oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(RAR 6913 kb)
Rights and permissions
About this article
Cite this article
Pan, W., Dong, H., Sun, R. et al. Plasma Ceramides in Relation to Coronary Plaque Characterization Determined by Optical Coherence Tomography. J. of Cardiovasc. Trans. Res. 14, 140–149 (2021). https://doi.org/10.1007/s12265-020-09978-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-020-09978-3