Abstract
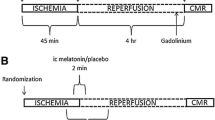
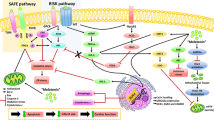
Melatonin has attenuated myocardial ischemia reperfusion injury in experimental studies. We hypothesized that the administration of melatonin during acute myocardial reperfusion improves myocardial salvage index in patients with ST-elevation myocardial infarction. Patients (n = 48) were randomized in a 1:1 ratio to intracoronary and intravenous melatonin (total 50 mg) or placebo. The myocardial salvage index assessed by cardiac magnetic resonance imaging at day 4 (± 1 day) after primary percutaneous coronary intervention was similar in the melatonin group (n = 22) at 55.3% (95% CI 47.0–63.6) and the placebo group (n = 19) at 61.5% (95% CI 57.5–65.5), p = 0.21. The levels of high-sensitive troponin T, creatinine kinase myocardial band, and oxidative biomarkers (advanced oxidation protein products, malondialdehyde, myeloperoxidase) were similar in the groups. The frequency of clinical events at 90 days did not differ between the groups. In conclusion, melatonin did not improve the myocardial salvage index after primary percutaneous coronary intervention in patients with ST elevation myocardial infarction compared with placebo.


Similar content being viewed by others
Abbreviations
- AAR:
-
Area at risk
- AUC:
-
Area under the curve
- AOPP:
-
Advanced oxidation protein products
- STEMI:
-
ST-elevation myocardial infarction
- CKMB:
-
Creatinine kinase myocardial band
- CMR:
-
Cardiac magnetic resonance imaging
- hs-TnT:
-
High-sensitive troponin T
- iqr:
-
Interquartile range
- LGE:
-
Late gadolinium enhancement images
- MDA:
-
Malondialdehyde
- MPO:
-
Myeloperoxidase
- MSI:
-
Myocardial salvage index
- NFE2L2:
-
Nuclear factor erythroid 2-like 2
- pPCI:
-
Primary percutaneous coronary intervention
- SSFP:
-
Steady-state free procession
- TIMI:
-
Thrombosis in myocardial infarction
- T2-STIR imaging:
-
Short-axis tau inversion recovery T2-weighted imaging
References
Goldberg, R. J., Currie, K., White, K., Brieger, D., Steg, P. G., Goodman, S. G., et al. (2004). Six-month outcomes in a multinational registry of patients hospitalized with an acute coronary syndrome (the Global Registry of Acute Coronary Events [GRACE]). The American Journal of Cardiology, 93(3), 288–293.
Larose, E., Rodes-Cabau, J., Pibarot, P., Rinfret, S., Proulx, G., Nguyen, C. M., et al. (2010). Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. Journal of the American College of Cardiology, 55(22), 2459–2469.
Lonborg, J., Vejlstrup, N., Kelbaek, H., Holmvang, L., Jorgensen, E., Helqvist, S., et al. (2013). Final infarct size measured by cardiovascular magnetic resonance in patients with ST elevation myocardial infarction predicts long-term clinical outcome: an observational study. European Heart Journal. Cardiovascular Imaging, 14(4), 387–395.
Burns, R. J., Gibbons, R. J., Yi, Q., Roberts, R. S., Miller, T. D., Schaer, G. L., et al. (2002). The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. Journal of the American College of Cardiology, 39(1), 30–36.
Yellon, D. M., & Hausenloy, D. J. (2007). Myocardial reperfusion injury. NEJM, 357(11), 1121–1135.
Frohlich, G. M., Meier, P., White, S. K., Yellon, D. M., & Hausenloy, D. J. (2013). Myocardial reperfusion injury: looking beyond primary PCI. European Heart Journal, 34(23), 1714–1722.
Reiter, R. J., Mayo, J. C., Tan, D. X., Sainz, R. M., Alatorre-Jimenez, M., & Qin, L. (2016). Melatonin as an antioxidant: under promises but over delivers. Journal of Pineal Research, 61(3), 253–278.
Reiter, R. J., Tan, D. X., Paredes, S. D., & Fuentes-Broto, L. (2010). Beneficial effects of melatonin in cardiovascular disease. Annals of Medicine, 42(4), 276–285.
Lochner, A., Genade, S., Davids, A., Ytrehus, K., & Moolman, J. A. (2006). Short- and long-term effects of melatonin on myocardial post-ischemic recovery. Journal of Pineal Research, 40(1), 56–63.
Genade, S., Genis, A., Ytrehus, K., Huisamen, B., & Lochner, A. (2008). Melatonin receptor-mediated protection against myocardial ischaemia/reperfusion injury: role of its anti-adrenergic actions. Journal of Pineal Research, 45(4), 449–458.
Dobsak, P., Siegelova, J., Eicher, J. C., Jancik, J., Svacinova, H., Vasku, J., et al. (2003). Melatonin protects against ischemia-reperfusion injury and inhibits apoptosis in isolated working rat heart. Pathophysiology, 9(3), 179–187.
Petrosillo, G., Colantuono, G., Moro, N., Ruggiero, F. M., Tiravanti, E., Di Venosa, N., et al. (2009). Melatonin protects against heart ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. American Journal of Physiology. Heart and Circulatory Physiology, 297(4), H1487–H1493.
Paradies, G., Petrosillo, G., Paradies, V., Reiter, R. J., & Ruggiero, F. M. (2010). Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. Journal of Pineal Research, 48(4), 297–310.
Halladin, N. L., Busch, S. E., Jensen, S. E., Hansen, H. S., Zaremba, T., Aaroe, J., et al. (2014). Intracoronary and systemic melatonin to patients with acute myocardial infarction: protocol for the IMPACT trial. Danish Medical Journal, 61(2), A4773.
Schulz, K. F., Altman, D. G., & Moher, D. (2011). CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery, 9(8), 672–677.
Rosenkilde, M. M., McLean, K. A., Holst, P. J., & Schwartz, T. W. (2004). The CXC chemokine receptor encoded by herpesvirus saimiri, ECRF3, shows ligand-regulated signaling through Gi, Gq, and G12/13 proteins but constitutive signaling only through Gi and G12/13 proteins. The Journal of Biological Chemistry, 279(31), 32524–32533.
Heydorn, A., Ward, R. J., Jorgensen, R., Rosenkilde, M. M., Frimurer, T. M., Milligan, G., et al. (2004). Identification of a novel site within G protein alpha subunits important for specificity of receptor-G protein interaction. Molecular Pharmacology, 66(2), 250–259.
Benned-Jensen, T., & Rosenkilde, M. M. (2008). Structural motifs of importance for the constitutive activity of the orphan 7TM receptor EBI2: analysis of receptor activation in the absence of an agonist. Molecular Pharmacology, 74(4), 1008–1021.
Hanasand, M., Omdal, R., Norheim, K. B., Goransson, L. G., Brede, C., & Jonsson, G. (2012). Improved detection of advanced oxidation protein products in plasma. Clinica Chimica Acta, 413(9–10), 901–906.
Yagi, K. (1998). Simple assay for the level of total lipid peroxides in serum or plasma. Methods in Molecular Biology, 108, 101–106.
Lykkesfeldt, J. (2001). Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: comparison with ultraviolet-visible spectrophotometry. Clinical Chemistry, 47(9), 1725–1727.
Seljeskog, E., Hervig, T., & Mansoor, M. A. (2006). A novel HPLC method for the measurement of thiobarbituric acid reactive substances (TBARS). A comparison with a commercially available kit. Clinical Biochemistry, 39(9), 947–954.
Lonborg, J., Vejlstrup, N., Kelbaek, H., Botker, H. E., Kim, W. Y., Mathiasen, A. B., et al. (2012). Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. European Heart Journal, 33(12), 1491–1499.
Tan, D. X., Manchester, L. C., Reiter, R. J., Qi, W., Kim, S. J., & El-Sokkary, G. H. (1998). Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: prevention by melatonin. Journal of Pineal Research, 25(3), 184–191.
Liu, L. F., Qin, Q., Qian, Z. H., Shi, M., Deng, Q. C., Zhu, W. P., et al. (2014). Protective effects of melatonin on ischemia-reperfusion induced myocardial damage and hemodynamic recovery in rats. European Review for Medical and Pharmacological Sciences, 18(23), 3681–3686.
Giacomo, C. G., & Antonio, M. (2007). Melatonin in cardiac ischemia/reperfusion-induced mitochondrial adaptive changes. Cardiovasc Hematol Dis Drug. Targets, 7(3), 163–169.
Petrosillo, G., Di Venosa, N., Pistolese, M., Casanova, G., Tiravanti, E., Colantuono, G., et al. (2006). Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia-reperfusion: role of cardiolipin. The FASEB Journal, 20(2), 269–276.
Diez, E. R., Renna, N. F., Prado, N. J., Lembo, C., Ponce Zumino, A. Z., Vazquez-Prieto, M., et al. (2013). Melatonin, given at the time of reperfusion, prevents ventricular arrhythmias in isolated hearts from fructose-fed rats and spontaneously hypertensive rats. Journal of Pineal Research, 55(2), 166–173.
Ekeløf, S. V., Halladin, N. L., Jensen, S. E., Zaremba, T., Aaroe, J., Kjaergaard, B., et al. (2016). Effects of intracoronary melatonin on ischemia-reperfusion injury in ST-elevation myocardial infarction. Heart and Vessels, 31(1), 88–95.
Gogenur, I., Kucukakin, B., Panduro Jensen, L., Reiter, R. J., & Rosenberg, J. (2014). Melatonin reduces cardiac morbidity and markers of myocardial ischemia after elective abdominal aortic aneurism repair: a randomized, placebo-controlled, clinical trial. Journal of Pineal Research, 57(3), 10–15.
Dwaich, K. H., Al-Amran, F. G., Al-Sheibani, B. I., & Al-Aubaidy, H. A. (2016). Melatonin effects on myocardial ischemia-reperfusion injury: Impact on the outcome in patients undergoing coronary artery bypass grafting surgery. International Journal of Cardiology, 221, 977–986.
Dominguez-Rodriguez, A., Abreu-Gonzalez, P., de la Torre-Hernandez, JM., Gonzalez-Gonzalez, J., Garcia-Camarero, T., Consuegra-Sanchez, L., et al. (2017). Effect of intravenous and intracoronary melatonin as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: results of the melatonin adjunct in the acute myocardial infarction treated with angioplasty trial. Journal of Pineal Research, 62(1). https://doi.org/10.1111/jpi.12374.
Galley, H. F., Lowes, D. A., Allen, L., Cameron, G., Aucott, L. S., & Webster, N. R. (2014). Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. Journal of Pineal Research, 56(4), 427–438.
Sahna, E., Parlakpinar, H., Turkoz, Y., & Acet, A. (2005). Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiological Research, 54(5), 491–495.
Sahna, E., Acet, A., Ozer, M. K., & Olmez, E. (2002). Myocardial ischemia-reperfusion in rats: reduction of infarct size by either supplemental physiological or pharmacological doses of melatonin. Journal of Pineal Research, 33(4), 234–238.
Andrabi, S. A., Sayeed, I., Siemen, D., Wolf, G., & Horn, T. F. (2004). Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. The FASEB Journal, 18(7), 869–871.
Engblom, H., Heiberg, E., Erlinge, D., Jensen, S. E., Nordrehaug, J. E., Dubois-Rande, J. L., et al. (2016). Sample size in clinical cardioprotection trials using myocardial salvage index, infarct size, or biochemical markers as endpoint. Journal of the American Heart Association, 5(3), e002708.
Carrick, D., Haig, C., Ahmed, N., Rauhalammi, S., Clerfond, G., Carberry, J., et al. (2016). Temporal evolution of myocardial hemorrhage and edema in patients after acute ST-segment elevation myocardial infarction: pathophysiological insights and clinical implications. Journal of the American Heart Association, 5(2).
Fernandez-Jimenez, R., Sanchez-Gonzalez, J., Aguero, J., Garcia-Prieto, J., Lopez-Martin, G. J., Garcia-Ruiz, J. M., et al. (2015). Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: imaging and histological tissue characterization. Journal of the American College of Cardiology, 65(4), 315–323.
Andersen, L. P., Gogenur, I., Rosenberg, J., & Reiter, R. J. (2016). Pharmacokinetics of melatonin: the missing link in clinical efficacy? Clinical Pharmacokinetics, 55(9), 1027–1030.
Serdar, Z., Serdar, A., Altin, A., Eryilmaz, U., & Albayrak, S. (2007). The relation between oxidant and antioxidant parameters and severity of acute coronary syndromes. Acta Cardiologica, 62(4), 373–380.
Lotfollahi, H., Mohammadi, M., Ghaffari, S., Badalzadeh, R., Sohrabi, B., Aslanabadi, N., et al. (2016). Effect of remote ischemic post-conditioning on oxidative stress in blood of STEMI patients treated with primary angioplasty. J Cardiovasc Thoracic Res, 8(3), 113–118.
Brennan, M. L., Penn, M. S., Van Lente, F., Nambi, V., Shishehbor, M. H., Aviles, R. J., et al. (2003). Prognostic value of myeloperoxidase in patients with chest pain. NEJM, 349(17), 1595–1604.
Vriend, J., & Reiter, R. J. (2015). The Keap1-Nrf2-antioxidant response element pathway: a review of its regulation by melatonin and the proteasome. Molecular and Cellular Endocrinology, 401, 213–220.
Guo, P., Pi, H., Xu, S., Zhang, L., Li, Y., Li, M., et al. (2014). Melatonin improves mitochondrial function by promoting MT1/SIRT1/PGC-1 alpha-dependent mitochondrial biogenesis in cadmium-induced hepatotoxicity in vitro. Toxicological Sciences, 142(1), 182–195.
Acknowledgements
We would like to thank the project nurses Kasper Villefrance and Charlotte Schmidt Skov, the PCI operators, and the staff in the catheterization laboratory at the Aalborg University Hospital.
Funding
This work was supported by grants from the University of Copenhagen, the Aase and Ejnar Danielsen’s Foundation, the Arvid Nilssons Foundation, the A P Møller Foundation for the Advancement of Medical Science, the Beckett Foundation, the Hede Nielsen Family Foundation, the Jens Anker Andersen Foundation, the Edith and Olfert Dines Hansen Foundation, the Axel Muusfeldts Foundation, the snedkermester Sophus Jacobsen and wife Astrid Jacobsen Foundation, and the Holger and Ruth Hesse Memorial Scholarship. None of the funders has had any influence on the study design, data collection, data analysis, interpretation, or writing of the manuscript. This study received no financial support from the industry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors
Additional information
Associate Editor Daniel P. Judge oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Ekeloef, S., Halladin, N., Fonnes, S. et al. Effect of Intracoronary and Intravenous Melatonin on Myocardial Salvage Index in Patients with ST-Elevation Myocardial Infarction: a Randomized Placebo Controlled Trial. J. of Cardiovasc. Trans. Res. 10, 470–479 (2017). https://doi.org/10.1007/s12265-017-9768-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-017-9768-7