Abstract
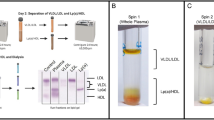
To assess cardiovascular risk in both clinical and basic research settings, it is imperative to be able to accurately measure plasma lipid levels. Here, methods commonly used to measure lipoproteins and lipids: ultracentrifugation (UC), fast protein liquid chromatography (FPLC), Roche auto-analyzer, and enzymatic assays were tested and compared. Plasma samples from 20 healthy humans and 22 cynomolgus monkeys were analyzed for their total cholesterol (TC), cholesterol in low density lipoproteins (LDL) and high density lipoproteins (HDL), and triglycerides (TG). Major lipid classes from UC and FPLC separated lipoprotein fractions from human plasma were further characterized by liquid chromatography-mass spectrometry analysis. All the tested methods showed acceptable performance with Roche analyzer among the best in approximate dilution linearity and recovery for most lipids as well as in repeatability between measurements of the same samples. TC, LDL, HDL, and TG values measured in human vs. monkey were—183.9 ± 35.5 (mean ± SD) vs. 105.6 ± 24.6 mg/dl, 106.0 ± 30.1 vs. 42.8 ± 13.0 mg/dl, 50.0 ± 11.4 vs. 53.4 ± 14.8 mg/dl, and 107.6 ± 50.7 vs. 58.0 ± 52.3 mg/dl. While no single method was uniformly the best, we recommend the Roche analyzer for routine measurements. UC or FPLC separation is needed for further functional characterization for specific lipid fraction. We have shown athero-protective profile in cynomolgus monkey compared with humans.





Similar content being viewed by others
References
Rader, D. J., & Daugherty, A. (2008). Translating molecular discoveries into new therapies for atherosclerosis. Nature, 451, 904–913.
da Luz, P. L., Favarato, D., Faria-Neto, J. R., Jr., Lemos, P., & Chagas, A. C. (2008). High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics (São Paulo, Brazil), 63, 427–432.
Hajer, G. R., van der Graaf, Y., Bots, M. L., Algra, A., & Visseren, F. L. (2009). Low plasma HDL-c, a vascular risk factor in high risk patients independent of LDL-c. European Journal of Clinical Investigation, 39, 680–688.
Gordon, T., Castelli, W. P., Hjortland, M. C., Kannel, W. B., & Dawber, T. R. (1977). High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. American Journal of Medicine, 62, 707–714.
Werner, R. M., & Pearson, T. A. (1998). LDL-cholesterol: a risk factor for coronary artery disease—From epidemiology to clinical trials. Canadian Journal of Cardiology, 14(Suppl B), 3B–10B.
Maron, D. J., Fazio, S., & Linton, M. F. (2000). Current perspectives on statins. Circulation, 101, 207–213.
van Dam, M., van Wissen, S., & Kastelein, J. J. (2002). Declaring war on undertreatment: Rationale for an aggressive approach to lowering cholesterol. Journal of Cardiovascular Risk, 9, 89–95.
Arca, M. (2007). Atorvastatin efficacy in the prevention of cardiovascular events in patients with diabetes mellitus and/or metabolic syndrome. Drugs, 67(Suppl 1), 43–54.
Havel, R. J., Eder, H. A., & Bragdon, J. H. (1955). The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. The Journal of Clinical Investigation, 34, 1345–1353.
Grundy, S. M. (2004). Richard Havel, Howard Eder, and the evolution of lipoprotein analysis. The Journal of Clinical Investigation, 114, 1034–1037.
Ordovas, J. M., & Osgood, D. (1998). Preparative isolation of plasma lipoproteins using fast protein liquid chromatography (FPLC). Methods in Molecular Biology, 110, 105–111.
Wiesner, P., Leidl, K., Boettcher, A., Schmitz, G., & Liebisch, G. (2009). Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. Journal of Lipid Research, 50, 574–585.
Miller, W. G., Myers, G. L., Sakurabayashi, I., Bachmann, L. M., Caudill, S. P., Dziekonski, A., et al. (2010). Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clinical Chemistry, 56, 977–986.
Yoshida, A., Naito, M., Kodama, M., & Nomura, H. (2001). Comparison of direct methods and HPLC for the measurement of HDL- and LDL-cholesterol with ultracentrifugation. Journal of Atherosclerosis and Thrombosis, 8, 84–90.
Egloff, M., Leglise, D., Duvillard, L., Steinmetz, J., Boyer, M. J., Ruelland, A., et al. (1999). Multicenter evaluation on different analyzers of three methods for direct HDL-cholesterol assay. Annales de Biologie Clinique, 57, 561–572.
Ordonez-Llanos, J., Wagner, A. M., Bonet-Marques, R., Sanchez-Quesada, J. L., Blanco-Vaca, F., & Gonzalez-Sastre, F. (2001). Which cholesterol are we measuring with the Roche direct, homogeneous LDL-C Plus assay? Clinical Chemistry, 47, 124–126.
Dansky, H. M., Shu, P., Donavan, M., Montagno, J., Nagle, D. L., Smutko, J. S., et al. (2002). A phenotype-sensitizing Apoe-deficient genetic background reveals novel atherosclerosis predisposition loci in the mouse. Genetics, 160, 1599–1608.
Castro-Perez, J. M., Kamphorst, J., DeGroot, J., Lafeber, F., Goshawk, J., & Yu, K. 2009. Comprehensive LC-MS E lipidomic analysis using a shotgun approach and its application to biomarker detection and identification in osteoarthritis patients. Journal of Proteome Research 9:2377–2389.
Bland, J. M., & Altman, D. G. (1986). Statistical methods for assessing agreement between two methods of clinical measurement. Lancet, 1, 307–310.
Warnick, G. R., Nauck, M., & Rifai, N. (2001). Evolution of methods for measurement of HDL-cholesterol: From ultracentrifugation to homogeneous assays. Clinical Chemistry, 47, 1579–1596.
Chen, Z., Strack, A.M., Stefanni, A.C., Chen, Y., Wu, W., Pan, Y., Urosevic-Price, O., Wang, L., McLaughlin, T., Geoghagen, N., et al., (2011). Validation of human ApoB and ApoAI immunoturbidity assays for non-human primate dyslipidemia and atherosclerosis research. J Cardiovasc Transl Res (in press)
Rudel, L. L. (1997). Genetic factors influence the atherogenic response of lipoproteins to dietary fat and cholesterol in nonhuman primates. Journal of the American College of Nutrition, 16, 306–312.
Suzuki, M., Yamamoto, D., Suzuki, T., Fujii, M., Suzuki, N., Fujishiro, M., et al. (2006). High fat and high fructose diet induced intracranial atherosclerosis and enhanced vasoconstrictor responses in non-human primate. Life Sciences, 80, 200–204.
Shively, C. A., & Clarkson, T. B. (1988). Regional obesity and coronary artery atherosclerosis in females: A non-human primate model. Acta Medica Scandinavica. Supplementum, 723, 71–78.
Yamada, T., Yoshikuni, Y., Taira, M., Yoshida-Suzuka, H., Kimura, K., Sakurai, I., et al. (1992). Diet-induced atherosclerosis in cynomolgus monkey aorta and regression by the sixth-month observation. Angiology, 43, 1008–1019.
Leblanc, M., Belanger, M. C., Julien, P., Tchernof, A., Labrie, C., Belanger, A., et al. (2004). Plasma lipoprotein profile in the male cynomolgus monkey under normal, hypogonadal, and combined androgen blockade conditions. Journal of Clinical Endocrinology and Metabolism, 89, 1849–1857.
Baron, B. W., Lyon, R. T., Zarins, C. K., Glagov, S., & Baron, J. M. (1990). Changes in plasma factor VIII complex and serum lipid profile during atherogenesis in cynomolgus monkeys. Arteriosclerosis, 10, 1074–1081.
Goldberg, I. J., Le, N. A., Paterniti, J. R., Jr., Ginsberg, H. N., Lindgren, F. T., & Brown, W. V. (1982). Lipoprotein metabolism during acute inhibition of hepatic triglyceride lipase in the cynomolgus monkey. The Journal of Clinical Investigation, 70, 1184–1192.
Acknowledgments
We would like to thank Christine Brzostowski for providing cynomolgus monkey plasma, Doree Gorden for collecting human plasma samples, and Dan Xi and Vinit Shah for LC/MS sample process and analysis. For disclosing conflict of interests, all authors are employees and may hold stocks or stock options of Merck & Co.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical relevance: Clinical trial has demonstrated that cardiovascular disease risk can be reduced through lipid-lowering therapy. Reliable laboratory measurements of plasma lipoproteins and lipid measurement are important in research investigations and clinical practice for lipid-lowering therapy and for assessing cardiovascular risks. This study provide comparability of measurement results using four common methodologies (ultracentrifugation, fast protein liquid chromatography, Roche auto-analyzer, and enzymatic assays) in healthy human and cynomolgus monkey plasma samples. We recommend the Roche analyzer for routine measurements while UC or FPLC separation is needed for further functional characterization for specific lipid fraction.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(PPT 115 kb)
Supplementary Table 2
(PPT 108 kb)
Supplementary Table 3
(PPT 101 kb)
Supplementary Figure 1
Comparison of methods by correlation and by Bland–Altman plot for human plasma. Linear regression lines and Bland–Altman plots for a total cholesterol (TC), b LDL cholesterol (LDL-C), c HDL cholesterol (HDL-C), and d TG in human plasma, comparing ultracentrifugation to each of the other three methods (PPT 302 kb)
Supplementary Figure 2
Comparison of methods by correlation and by Bland–Altman plots for cynomolgus plasma. Linear regression lines and Bland–Altman plots for a total cholesterol (TC), b LDL cholesterol (LDL-C), c HDL cholesterol (HDL-C), and d TG in cynomolgus plasma, comparing ultracentrifugation to each of the other three methods (PPT 311 kb)
Supplementary Figure 3
Relation of cholesterol in LDL or HDL and apolipoproteins. LDL cholesterol, HDL cholesterol, apoB, and apoA1 in cynomolgus plasma samples were determined using Roche analyzer. a Plot of plasma LDL cholesterol concentration against apoB level. b Plot of plasma HDL cholesterol concentration against apoA1 level (PPT 83 kb)
Rights and permissions
About this article
Cite this article
Han, S., Flattery, A.M., McLaren, D. et al. Comparison of Lipoprotein Separation and Lipid Analysis Methodologies for Human and Cynomolgus Monkey Plasma Samples. J. of Cardiovasc. Trans. Res. 5, 75–83 (2012). https://doi.org/10.1007/s12265-011-9340-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-011-9340-9