Abstract
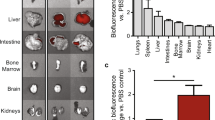
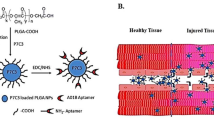
An estimated 985,000 new myocardial infarctions will occur in the USA in 2011. While many will survive the initial insult, the early damage will eventually lead to heart failure for which the only definitive cure is transplantation. Cardiomyocyte (CM) apoptosis is a large contributor to cardiac dysfunction, and although potential therapeutic molecules exist to inhibit apoptotic pathways, drug delivery methods are lacking. This damage is largely regional and thus localized delivery of therapeutics holds great potential; however, CMs are relatively non-phagocytic, which limits existing options that rely on phagocytosis. Recently, the sugar N-acetylglucosamine (GlcNAc) was shown to be bound and internalized by CMs, providing a potential mechanism for drug delivery. Here we demonstrate efficacy of a drug delivery system comprising a drug-loaded biodegradable polyketal nanoparticle that is surface-decorated with GlcNAc. Inclusion of the sugar enhanced uptake by CMs as measured by intracellular activated fluorescence. When delivered in vivo following ischemia–reperfusion injury, GlcNAc-decorated particles loaded with the p38 inhibitor SB239063 reduced apoptotic events and infarct size and improved acute cardiac function. This was in contrast to our published data demonstrating no acute effect of non-sugar-decorated, p38 inhibitor-loaded particles. These data suggest a novel therapeutic option to enhance uptake of drug-loaded nanoparticles to CMs and perhaps reduce the large amount of CM cell death following myocardial injury.







Similar content being viewed by others
References
Lloyd-Jones, D., Adams, R. J., Brown, T. M., Carnethon, M., Dai, S., De Simone, G., et al. (2010). Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation, 121(7), e46.
Maulik, N., Yoshida, T., & Das, D. K. (1998). Oxidative stress developed during the reperfusion of ischemic myocardium induces apoptosis. Free Radical Biology & Medicine, 24(5), 869–875.
Bialik, S., Geenen, D. L., Sasson, I. E., Cheng, R., Horner, J. W., Evans, S. M., et al. (1997). Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. The Journal of Clinical Investigation, 100(6), 1363.
McGill, C. J., & Brooks, G. (1995). Cell cycle control mechanisms and their role in cardiac growth. Cardiovascular Research, 30(4), 557–569.
Rumyantsev, P. P. (1977). Interrelations of the proliferation and differentiation processes during cardiac myogenesis and regeneration. International Review of Cytology, 51, 187–273.
Kajstura, J., Leri, A., Finato, N., Di Loreto, C., Beltrami, C. A., & Anversa, P. (1998). Myocyte proliferation in end-stage cardiac failure in humans. Proceedings of the National Academy of Sciences of the United States of America, 95(15), 8801.
Garg, S., Narula, J., & Chandrashekhar, Y. (2005). Apoptosis and heart failure: Clinical relevance and therapeutic target. Journal of Molecular and Cell Cardiology, 38(1), 73–79.
Park, M., Shen, Y. T., Gaussin, V., Heyndrickx, G. R., Bartunek, J., Resuello, R. R. G., et al. (2009). Apoptosis predominates in nonmyocytes in heart failure. American Journal of Physiology. Heart and Circulatory Physiology, 297(2), H785.
Buja, L. M., & Vela, D. (2008). Cardiomyocyte death and renewal in the normal and diseased heart. Cardiovascular Pathology, 16(6), 349–374. doi:10.1016/j.carpath.2008.02.004.
Hockenbery, D. M., Oltvai, Z. N., Yin, X. M., Milliman, C. L., & Korsmeyer, S. J. (1993). Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell, 75(2), 241–251.
Maulik, N., Engelman, R. M., Rousou, J. A., Flack, J. E., III, Deaton, D., & Das, D. K. (1999). Ischemic preconditioning reduces apoptosis by upregulating anti-death gene Bcl-2. Circulation, 100(90002), II–369.
Huang, J., Ito, Y., Morikawa, M., Uchida, H., Kobune, M., Sasaki, K., et al. (2003). Bcl-xL gene transfer protects the heart against ischemia/reperfusion injury. Biochemical and Biophysical Research Communications, 311(1), 64–70.
Potts, M. B. (2005). Reduced Apaf-1 levels in cardiomyocytes engage strict regulation of apoptosis by endogenous XIAP. The Journal of Cell Biology, 171(6), 925–930. doi:10.1083/jcb.200504082.
Jolly, S., Kane, W., Bailie, M., Abrams, G., & Lucchesi, B. (1984). Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circulation Research, 54(3), 277.
Khaper, N., Kaur, K., Li, T., Farahmand, F., & Singal, P. (2003). Antioxidant enzyme gene expression in congestive heart failure following mycardial infarction. Molecular and Cellular Biochemistry, 251(1), 9–15.
Andreka, P., Zang, J., Dougherty, C., Slepak, T. I., Webster, K. A., & Bishopric, N. H. (2001). Cytoprotection by Jun kinase during nitric oxide-induced cardiac myocyte apoptosis. Circulation Research, 88(3), 305.
Minamino, T., Yujiri, T., Papst, P. J., Chan, E. D., Johnson, G. L., & Terada, N. (1999). MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proceedings of the National Academy of Sciences of the United States of America, 96(26), 15127.
Franke, T. F., Kaplan, D. R., & Cantley, L. C. (1997). PI3K: Downstream AKTion blocks apoptosis. Cell, 88(4), 435.
Wang, Y., Huang, S., Sah, V. P., Ross, J., Brown, J. H., Han, J., et al. (1998). Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. Journal of Biological Chemistry, 273(4), 2161.
Chen, Z., Chua, C. C., Ho, Y. S., Hamdy, R. C., & Chua, B. H. L. (2001). Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. American Journal of Physiology. Heart and Circulatory Physiology, 280(5), H2313.
Chen, Z., Siu, B., Ho, Y. S., Vincent, R., Chua, C. C., Hamdy, R. C., et al. (1998). Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. Journal of Molecular and Cell Cardiology, 30(11), 2281–2289.
Chua, C. C., Gao, J., Ho, Y. S., Xiong, Y., Xu, X., Chen, Z., et al. (2007). Overexpression of IAP-2 attenuates apoptosis and protects against myocardial ischemia/reperfusion injury in transgenic mice. Biochimica et Biophysica Acta, 1773(4), 577–583.
Matherne, G. P., Linden, J., Byford, A. M., Gauthier, N. S., & Headrick, J. P. (1997). Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proceedings of the National Academy of Sciences of the United States of America, 94(12), 6541.
Matsui, T., Tao, J., del Monte, F., Lee, K. H., Li, L., Picard, M., et al. (2001). Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation, 104(3), 330.
Harris, J. M., & Chess, R. B. (2003). Effect of pegylation on pharmaceuticals. Nature Reviews. Drug Discovery, 2(3), 214–221.
Hsieh, P. C. H., Davis, M. E., Gannon, J., MacGillivray, C., & Lee, R. T. (2006). Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. The Journal of Clinical Investigation, 116(1), 237–248.
Lee, S., & Murthy, N. (2007). Targeted delivery of catalase and superoxide dismutase to macrophages using folate. Biochemical and Biophysical Research Communications, 360(1), 275–279.
Lee, S., Yang, S. C., Heffernan, M. J., Taylor, W. R., & Murthy, N. (2007). Polyketal microparticles: A new delivery vehicle for superoxide dismutase. Bioconjugate Chemistry, 18(1), 4–7.
Sy, J., Phelps, E., García, A., Murthy, N., & Davis, M. (2010). Surface functionalization of polyketal microparticles with nitrilotriacetic acid–nickel complexes for efficient protein capture and delivery. Biomaterials, 31(18), 4987–4994.
Sy, J., Seshadri, G., Yang, S., Brown, M., Oh, T., Dikalov, S., et al. (2008). Sustained release of a p38 inhibitor from non-inflammatory microspheres inhibits cardiac dysfunction. Nature Materials, 7(11), 863–868.
Sutton, M. G., & Sharpe, N. (2000). Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy. Circulation, 101(25), 2981.
Aso, S., Ise, H., Takahashi, M., Kobayashi, S., Morimoto, H., Izawa, A., et al. (2007). Effective uptake of N-acetylglucosamine-conjugated liposomes by cardiomyocytes in vitro. Journal of Controlled Release, 122(2), 189–198.
Ise, H., Kobayashi, S., Goto, M., Sato, T., Kawakubo, M., Takahashi, M., et al. (2010). Vimentin and desmin possess GlcNAc-binding lectin-like properties on cell surfaces. Glycobiology, 20(7), 843.
Vemuri, S., & Rhodes, C. (1995). Preparation and characterization of liposomes as therapeutic delivery systems: A review. Pharmaceutica Acta Helvetiae, 70(2), 95–111.
Seshadri, G., Sy, J. C., Brown, M., Dikalov, S., Yang, S. C., Murthy, N., et al. (2010). The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia–reperfusion injury. Biomater, 31(6), 1372–1379.
Yuan, X. B., Gu, M. Q., Kang, C. S., Zhao, Y. H., Tian, N. J., Pu, P. Y., et al. (2007). Surface biofunctionalization of PLA nanoparticles through amphiphilic polysaccharide coating and ligand coupling: Evaluation of biofunctionalization and drug releasing behavior. Carbohydrate Polymers, 67(3), 417–426.
Granger, B. L., & Lazarides, E. (1979). Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell, 18(4), 1053–1063.
Li, Z., Mericskay, M., Agbulut, O., Butler-Browne, G., Carlsson, L., Thornell, L. E., et al. (1997). Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. The Journal of Cell Biology, 139(1), 129–144.
Bogoyevitch, M. A., Gillespie-Brown, J., Ketterman, A. J., Fuller, S. J., Ben-Levy, R., Ashworth, A., et al. (1996). Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circulation Research, 79(2), 162–173.
Pombo, C. M., Bonventre, J. V., Avruch, J., Woodgett, J. R., Kyriakis, J. M., & Force, T. (1994). The stress-activated protein kinases are major c-Jun amino-terminal kinases activated by ischemia and reperfusion. Journal of Biological Chemistry, 269(42), 26546–26551.
Yin, T., Sandhu, G., Wolfgang, C. D., Burrier, A., Webb, R. L., Rigel, D. F., et al. (1997). Tissue specific pattern of stress kinase activation in ischemia/reperfused heart and kidney. Journal of Biological Chemistry, 272, 19943–19950.
Amado, L. C., Saliaris, A. P., Schuleri, K. H., St John, M., Xie, J. S., Cattaneo, S., et al. (2005). Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America, 102(32), 11474–11479.
Krause, K., Jaquet, K., Schneider, C., Haupt, S., Lioznov, M. V., Otte, K. M., et al. (2009). Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: First-in-man study. Heart, 95(14), 1145–1152.
Herreros, J., Prosper, F., Perez, A., Gavira, J. J., Garcia-Velloso, M. J., Barba, J., et al. (2003). Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. European Heart Journal, 24(22), 2012–2020.
Li, Q., Li, B., Wang, X., Leri, A., Jana, K. P., Liu, Y., et al. (1997). Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. The Journal of Clinical Investigation, 100(8), 1991–1999.
Sabbah, H. N., Sharov, V. G., Gupta, R. C., Todor, A., Singh, V., & Goldstein, S. (2000). Chronic therapy with metoprolol attenuates cardiomyocyte apoptosis in dogs with heart failure. Journal of the American College of Cardiology, 36(5), 1698–1705.
Jones, S. P., Zachara, N. E., Ngoh, G. A., Hill, B. G., Teshima, Y., Bhatnagar, A., et al. (2008). Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation, 117(9), 1172.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, as a Program of Excellence in Nanotechnology Award, N01 HV-08234, to NM and MED. Additionally, this work was supported by award HL090601 from the National Institutes of Health to MED, as well as a GAANN Fellowship from the Center for Drug Design, Development, and Delivery at Georgia Institute of Technology to WDG.
Conflict of Interest Statement
Drs. Davis and Murthy, as well as Emory University, are entitled to equity and royalties derived from Ketal Biomedical Incorporated, which is developing products related to the technology described in this paper. This study could affect his/her/their personal financial status. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gray, W.D., Che, P., Brown, M. et al. N-acetylglucosamine Conjugated to Nanoparticles Enhances Myocyte Uptake and Improves Delivery of a Small Molecule p38 Inhibitor for Post-infarct Healing. J. of Cardiovasc. Trans. Res. 4, 631–643 (2011). https://doi.org/10.1007/s12265-011-9292-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-011-9292-0