Abstract
Very small embryonic-like cells (VSELs) are a population of stem cells residing in the bone marrow (BM) and several organs, which undergo mobilization into peripheral blood (PB) following acute myocardial infarction and stroke. These cells express markers of pluripotent stem cells (PSCs), such as Oct-4, Nanog, and SSEA-1, as well as early cardiac, endothelial, and neural tissue developmental markers. VSELs can be effectively isolated from the BM, umbilical cord blood, and PB. Peripheral blood and BM-derived VSELs can be expanded in co-culture with C2C12 myoblast feeder layer and undergo differentiation into cells from all three germ layers, including cardiomyocytes and vascular endothelial cells. Isolation of VSLEs using fluorescence-activated cell sorting multiparameter live cell sorting system is dependent on gating strategy based on their small size and expression of PSC and absence of hematopoietic lineage markers. VSELs express early cardiac and endothelial lineages markers (GATA-4, Nkx2.5/Csx, VE-cadherin, and von Willebrand factor), SDF-1 chemokine receptor CXCR4, and undergo rapid mobilization in acute MI and ischemic stroke. Experiments in mice showed differentiation of BM-derived VSELs into cardiac myocytes and effectiveness of expanded and pre-differentiated VSLEs in improvement of left ventricular ejection fraction after myocardial infarction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid progress in the field of experimental studies on cardiovascular regeneration is being translated to the clinical application of stem cells (SC) isolated from the bone marrow (BM) or the myocardium (cardiac stem cells, CSC). Aim of this approach is to promote the myocardial recovery in patients with acute myocardial infarction (MI) or to improve the cardiac function in the setting of ischemic cardiomyopathy. So far, there is no proof that use of SC can lead to bona fide cardiac regeneration and the mechanism of beneficial effects observed in some studies is probably mediated by paracrine effects leading to neoangiogenesis, reduction of apoptosis, as well as recruitment of CSC to the site of the ischemic injury [1]. At the current state of clinical application of SC, there is no convincing data showing the superiority of any particular-type cells or their source, so heterogenous population of BM-derived mononuclear cells (MNC) is used most often; however, some recent studies assess the efficiency of selected subpopulations of BMC, such as CD133+, CD34 + CXCR4+ cells, mesenchymal stromal cells (MSC), or CSC [2].
Despite the encouraging experience from trials using BM-derived MNC novel types of SC carrying higher reparatory potential are clearly needed. Such populations include CSC [3], engineered BM-derived progenitor cells (e.g., C-Cure) [4], allogeneic MSC [5], and pluripotent stem cells (PSC). PSC can be produced using gene transfer (induced pluripotent stem cells) [6, 7] or isolated from the adult tissues (very small embryonic-like stem cells [VSELs] [8]). Isolation of PSC from adult tissues seems to be very promising approach because cells obtained in such way are ethically acceptable, however efficient methods of isolation and expansion in culture of human cells are still not available [9, 10]. This review discusses the recent data on characteristics and potential clinical application of VSELs.
Potential Role of PSC, Including VSELs in Adult Organisms
Adult tissue PSC represent a population of epiblast-derived progeny which survive into adulthood in different locations in BM and solid organs. We hypothesized that these cells, including VSELs, migrate during embryogenesis along with hematopoietic stem cells (HSCs) to the BM, and their migration follows the gradient of chemoattractants, including chemokine stromal cell-derived factor-1 (SDF-1) [11]. Their potential role is to be a reserve population of SC and tissue-committed progenitor cells which can be mobilized after tissue injury. VSELs are primitive cells expressing the markers typical for primordial germ cells including Stella, Fragilis, Nobox, Hdac6, and CXCR4. We hypothesize that quiescent VSELs serve as a reserve pool of PSC and are part of the physiological mechanism of tissue repair and renewal of resident SC [11]. Their quiescence is a safety mechanism preventing the formation of teratomas.
Isolation and Sources of VSELs
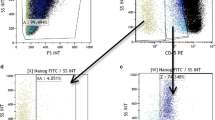
Initially, rare population of VSELs was isolated from adult murine BM by Kucia et al. by multiparameter fluorescence-activated cell sorting (FACS). Our group established the criteria for sorting of VSELs based on the presence of several surface markers and the diameter of the cells. Figure 1 shows the gating strategy used for FACS sorting. The detailed description of the protocol was published elsewhere. Briefly, the initial step is the lysis of red blood cells to obtain the fraction of nucleated cells. Erythrocyte lysis buffer is used instead of Ficoll centrifugation because the latter approach might deplete the population of very small cells [12].
Strategy for isolation of VSELs from human peripheral blood after mobilization with C-CSF (MPB-VSELs) using FACS-based live cell sorting system. Gating strategy was developed by using of the synthetic beads of known diameters (1, 2, 4, 6, 10, 15 μm) (a) to define the extended lymph-gate for subsequent sorting (b). After lysis of erythrocytes, mobilized peripheral blood total nucleated cells (TNCs) fraction is stained with antibodies against hematopoietic lineages markers (Lin), CD133 stem cell antigen (c), and CD45 pan-leukocytic antigen (d). Gate R1 was set up to include objects with diameter > 2 μm. Events included in region R1 were analyzed for presence of hematopoietic lineages markers. Subsequently, only lin− events were included into region R2, and cells expressing CD133 antigens were further isolated depending on the presence of CD45 antigen (gates R3-4). VSELs are lin−CD45-CD133+ cells (gate R3) whereas hematopoietic stem cells (HPCs) are included in gate R4 and constitute population of lin−CD45+CD133+
Subsequently, cells are stained with antibodies against Sca-1 (murine VSELs) or CD133 (human VSELs), pan-hematopoietic antigen (CD45), hematopoietic lineages markers (lin), and CXCR4 and sorted using a multiparameter, live sterile cell sorting systems (MoFlo, Beckman Coulter; FACSAria, Beckton Dickinson) [8]. We used “extended lymphocyte gate” to include events with diameter 2–10 μm, including approximately 95% of VSELs. The width of the gate was validated by using synthetic beads of predefined size (1–15 μm) [12]. Proper definition of the gate is crucial because this area of the cytogram includes not only cells, but also cellular debris [13, 14]. Several other approaches to define the population of small cells were used, including ImageStream system, which is a combination of FACS and immunofluorescent (IF) microscope and allows to “decode” any particular events on cytogram and visualize it to confirm morphology and presence of markers consistent with VSELs phenotype [13, 14]. VSELs were so far isolated in mice (BM, peripheral blood, fetal liver, and several solid organs in adult animals including brain, heart, retina, kidneys, pancreas, skeletal muscles spleen, and thymus) and humans [15]. In humans, VSELs were successfully isolated from umbilical cord blood (UCB) and peripheral blood (PB). Currently, our group investigates the presence of VSELs in adult BM and myocardium [16, 17].
Structural, Molecular, and Functional Characteristics of Freshly Isolated VSELs
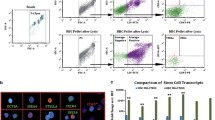
VSELs confer a very small population of cells, and their number is as low as 0.030 ± 0.008% of total BM cells in mice. Both murine BM-derived as well as human PB-derived VSELs have significantly smaller diameter than monocytes and granulocytes, and are larger than platelets [4, 5]. In addition, some differences between murine and human VSELs were observed. Human UCB- and PB-derived VSELs consist of lin−CD45-CXCR4 + CD133+ and consistently with differences in mean size of leukocytes and RBC between humans and mice are larger than murine (~6–7 μm) [13]. Population of Sca-1+lin−CD45− cells was enriched for PSC markers, such as Oct-4, Nanog, SSEA-1, Rex1, and Dppa3, Rif-1. In addition, VSELs express CXCR4 and migrated to the SDF-1 gradient. Several imaging tools were used to define the morphology of VSELs on the single cell level. Studies using transmission electron microscopy confirmed that VSELs differed in several aspects from HSC. As showed on Fig. 2, VSELs are significantly smaller than HSCs (3–6 vs. 6–8 μm) and have higher nucleus/cytoplasm ratio. Nucleus is large, contains open-type chromatin and is surrounded by the narrow rim of cytoplasm with numerous mitochondria. Therefore, their morphology is consistent with primitive PSC [8, 18, 19]. VSELs display non-hematopoietic immunophenotype (lin−CD45−) and do not offer radioprotection in lethally irradiated recipient mice. Freshly isolated and expanded VSELs do not form hematopoietic colonies in vitro [13, 20]. Distinct immunophenotype and size are major criteria for isolation of VSELs, and the presence of PSC markers was confirmed using real-time RT-PCR, at protein level by IF staining and ImageStream system. Importantly, because of the possibility of the detection of pseudogenes raised by some investigators, our group recently demonstrated that the promoters of Oct-4 and Nanog in VSELs contain transcriptionally active chromatin [21]. Importantly, VSELs and HSCs are different not only in terms of their morphology.
Representative images of human peripheral blood-derived VSEL and HSPC by ImageStreamX system. Human blood cells were stained for markers distinguishing VSELs such as: (1) CD45 pan-leukocytic antigen (APC-Cy7, cyan), (2) hematopoietic lineages markers (FITC, green) and (3) stem cell antigens CD133 (PE, yellow), and CD34 (APC, violet). Nuclei were stained with Hoechst 33342 dye. Images were collected by imaging flow cytometer-ImageStreamX system. VSELs and HSPCs were distinguished based on CD45 antigen expression
Freshly isolated VSELs were expanded in co-culture with C2C12 myoblast feeder layer and after 7–10 days, formed sphere-like clusters consisting of a few hundred cells resembling embryoid bodies (VSEL-derived spheres, VSEL-DSs). VSEL-DSs expressed placenta-like alkaline phosphatase. Co-culture allowed for expansion of VSELs, and after isolation of expanded VSELs, we demonstrated their capacity to differentiate into cell lines from all three germ layers, such as mesodermal cardiomyocytes, ectodermal neural cells, and endodermal pancreatic cells [22]. Importantly, the pluripotent features of VSELs are retained in the population of mobilized to PB murine VSELs. Such circulating cells showed expression of PSC markers on the level similar to ES-D3 murine embryonic cells. Such observations support the hypothesis that mobilized cells might contribute to tissue repair because of their broad differentiation capacity [18].
VSELs display morphological and molecular features of PSCs. Several molecular markers are consistent with PSC phenotype, such as Oct4 and Nanog, presence of bivalent domains in promoters of developmentally important transcription factors (Sox21, Nkx2.2, Dlx1, Lbx14, Hlx9), and partially reactivate inactivated the X chromosome in female PSCs. VSELs differentiate into cells from all three germ layers. Importantly, the pluripotent features observed in murine cells were not demonstrated in human VSELs. In fact, VSELs neither fulfill the criteria for blastocyst complementation nor show the ability to form teratomas in immunodeficient mice. The quiescence of VSELs isolated from adult tissue can be explained by their quiescence related to epigenetic modification of crucial imprinted genes, such as Igf2 and RasGRF1. Quiescence is probably a mechanism of prevention against the formation of teratomas [21].
Mobilization, Circulation, and Homing of VSELs
Bone marrow-derived SC must undergo rapid mobilization in order to participate in tissue repair. In addition, there must be a variety of chemical signals to which the VSELs must respond to orchestrate their homing and engraftment into the ischemic or otherwise damaged tissue (irradiation, burns) [23]. Data from animal studies showed rapid mobilization of SC from the BM to the peripheral blood, as evidenced by an increase of circulating cells enriched with early cardiac markers (GATA-4 and Nkx2.5/Csx), which migrated along the SDF-1 gradient [24, 25].
Acute Myocardial Infarction
Acute MI leads to a generalized inflammatory response with increased production and release of inflammatory markers, and also of chemoattractant factors such as kinins, chemokines, cytokines, and growth factors, components of the complement cascade. This coexists with subsequent mobilization of SC and endothelial progenitor cells as well as leukocytes [24, 26–28].
In healthy humans, a very small number of VSELs [0.8–1.3 cells/μL] can be detected reflecting continuous efflux of SC from the BM [10, 25, 28]. During acute MI, the number of VSELs is significantly higher and the expression of mRNA of PSC and cardiac markers is up-regulated [25]. Numerous hematopoietic and inflammatory cytokines known to regulate mobilization of SC are up-regulated in acute coronary syndromes. Several factors involved in trafficking of VSELs are SDF-1, leukemia inhibitory factor, hepatocyte growth factor, and stem cell factor-CD117 axes [24, 29, 30]. Figure 3 shows the mechanisms of mobilization and homing of VSELs in acute MI.
The putative mechanism of mobilization and homing of VSELs in acute myocardial infarction. Myocardial ischemia induces increased expression of chemoattractants [chemokines (SDF-1), growth factors (VEGF, HGF), cytokines (LIF)] and release of phospholipids predominantly in infarct border zone. The increased expression of chemoattractants in the ischemic organ creates the reversal of chemoattractant gradient leading to the release of VSELs from the bone marrow niches and their homing to the site of the ischemic injury. Within the bone marrow niche, the mobilization of VSELs is orchestrated by expression of matrix metaloproteinases and activation of complement cascade. Also, phospholipids, such as sphingosine-1-phosphate influence the mobilization of VSELs. In peripheral blood in healthy subjects, VSELs can be detected at a very low number. After ischemic injury, these cells are rapidly mobilized into peripheral blood and the expression of pluripotent stem cell markers, as well as early cardiac, endothelial, muscle, and neural markers is significantly increased. Hence, we hypothesized that mobilization of VSELs is a part of reparatory mechanism activated in the setting of acute myocardial infarction. Also, circulation of VSELs might contribute to the pool of resident cardiac stem cells
The number of mobilized VSELs is dependent on several factors, such as age, presence of diabetes, and the extent of myocardial damage [18]. In our hands, mobilization of VSELs was reduced in elderly and diabetic patients with acute MI [31]. Also, patients with more severely impaired cardiac function (reduced left ventricular ejection fraction, high levels of cardiac troponins) had reduced number of circulating cells [25, 32].
Ischemic Stroke
Similar to acute MI, ischemic stroke was associated with significant increase of the number of circulating VSELs in PB. These cells expressed increased levels of mRNA for neural markers (GFAP, nestin, beta-III-tubulin, Olig1, Olig2, Sox2, and Musashi) as well as PSC markers (Oct-4, Nanog). This suggests that mobilization of VSELs is an important mechanism in different forms of tissue ischemia [33].
Physical Exercise
Physical exercise, especially a regular one, was shown to increase the number and improve the function of circulating SC and progenitor cells. Our preliminary data showed that intensive treadmill exercise induced the transient mobilization of VSELs into peripheral blood. An increased number of VSELs was detected as early as after the exercise, and circulating cells showed increased expression of mRNA for PSC (Oct-4, Nanog), cardiac (GATA-4, Nkx2.5/Csx, MEF2C), and endothelial markers (VE-cadherin). Mobilization was negatively correlated with an extent of coronary artery disease in angiography (one- vs. two- and three-vessel disease) [submitted].
Potential Application of VSELs and VSEL-Enriched Populations in Clinical Trials
The measurement of the number of VSELs might, after validation in larger population, serve as a prognostic marker in acute MI because the preliminary data showed inverse correlation with the extent of myocardial injury and presence of co-morbidities, such as diabetes. Another approach is to use the cells for prevention of left ventricular dysfunction after MI. We developed a protocol for expansion and differentiation of BM-derived VSELs into cardiac myocytes. The differentiation of murine VSEL-derived cardiac myocytes (CM) closely resembles the same process in embryonic stem cell-derived CM [8, 34]. Dawn et al. showed that direct intramyocardial injection of freshly isolated VSELs in mice with reperfused MI improved global and regional left ventricular contractility. Moreover, the beneficial effects were observed after 35 days of follow-up. The histopathology study showed rare VSEL-derived cardiac myocytes in the recipients' heart muscle [35]. Additionally, expansion and pre-differentiation of VSELs in cardiopoiesis-guided media for 5 days before the injection increased their effectiveness leading to increase of left ventricular ejection fraction, myocardial systolic thickening, and attenuated remodeling after 35 days post-MI [36]. Clinical studies using autologous VSELs are needed to validate these promising experimental data, providing that clinically approved protocols for cell expansion and differentiation are available. Several other emerging stem cell technologies such as CSC, genetically engineered bone marrow progenitor cells (e.g., EPC overexpressing endothelial nitric oxide synthase), allogeneic bone marrow-derived MSC and cardiopoiesis-guided bone marrow-derived mesenchymal cardiopoietic cells are under translation into clinical use.
References
Abdel-Latif, A., Bolli, R., Tleyjeh, I. M., Montori, V. M., Perin, E. C., Hornung, C. A., et al. (2007). Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Archives of Internal Medicine, 167(10), 989–997.
Dimmeler, S., Burchfield, J., & Zeiher, A. M. (2008). Cell-based therapy of myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology, 28(2), 208–216.
Messina, E., De Angelis, L., Frati, G., Morrone, S., Chimenti, S., Fiordaliso, F., et al. (2004). Isolation and expansion of adult cardiac stem cells from human and murine heart. Circulation Research, 95(9), 911–921.
Behfar, A., Yamada, S., Crespo-Diaz, R., Nesbitt, J. J., Rowe, L. A., Perez-Terzic, C., et al. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. Journal of the American College of Cardiology, 569, 721–734.
Pittenger, M. F., & Martin, B. J. (2004). Mesenchymal stem cells and their potential as cardiac therapeutics. Circulation Research, 95(1), 9–20.
Martinez-Fernandez, A., Nelson, T. J., Yamada, S., Reyes, S., Alekseev, A. E., Perez-Terzic, C., et al. (2009). iPS programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circulation Research, 105(7), 648–656.
Nelson, T. J., Martinez-Fernandez, A., & Terzic, A. (2010). Induced pluripotent stem cells: developmental biology to regenerative medicine. Nature Reviews in Cardiology, 7(12), 700–710.
Kucia, M., Reca, R., Campbell, F. R., Zuba-Surma, E., Majka, M., Ratajczak, J., et al. (2006). A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia, 20(5), 857–869.
Dimmeler, S. Regulation of bone marrow-derived vascular progenitor cell mobilization and maintenance. Arteriosclerosis, Thrombosis, and Vascular Biology, 306, 1088–1093.
Tang, X. L., Rokosh, D. G., Guo, Y., & Bolli, R. (2010). Cardiac progenitor cells and bone marrow-derived very small embryonic-like stem cells for cardiac repair after myocardial infarction. Circulation Journal, 74(3), 390–404.
Ratajczak, M. Z., Machalinski, B., Wojakowski, W., Ratajczak, J., & Kucia, M. (2007). A hypothesis for an embryonic origin of pluripotent Oct-4+ stem cells in adult bone marrow and other tissues. Leukemia, 21, 860–867.
Zuba-Surma, E. K., Kucia, M., Abdel-Latif, A., Dawnn, B., Hall, B., Singh, R., et al. (2008). Morphological characterization of very small embryonic-like stem cells (VSELs) by ImageStream system analysis. Journal of Cellular and Molecular Medicine, 12(1), 292–303.
Zuba-Surma, E. K., & Ratajczak, M. Z. (2010). Overview of very small embryonic-like stem cells (VSELs) and methodology of their identification and isolation by flow cytometric methods. Current Protocols in Cytometry, Chapter 9, Unit 9.29.
Zuba-Surma, E. K., Kucia, M., Wu, W., Klich, I., Lillard, J. W., Jr., Ratajczak, J., et al. (2008). Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytometry. Part A, 73A(12), 1116–1127.
Zuba-Surma, E. K., Kucia, M., Rui, L., Shin, D. M., Wojakowski, W., Ratajczak, J., et al. (2009). Fetal liver very small embryonic/epiblast like stem cells follow developmental migratory pathway of hematopoietic stem cells. Annals of the New York Academy of Sciences, 1176, 205–218.
Ratajczak, M. Z., Kucia, M., Ratajczak, J., & Zuba-Surma, E. K. (2009). A multi-instrumental approach to identify and purify very small embryonic like stem cells (VSELs) from adult tissues. Micron, 40(3), 386–393.
Ratajczak, M. Z., Zuba-Surma, E. K., Machalinski, B., Ratajczak, J., & Kucia, M. (2008). Very small embryonic-like (VSEL) stem cells: purification from adult organs, characterization, and biological significance. Stem Cell Reviews, 4(2), 89–99.
Kucia, M., Wysoczynski, M., Wu, W., Zuba-Surma, E., Ratajczak, J., & Ratajczak, M. Z. (2008). Evidence that very small embryonic-like stem cells are mobilized into peripheral blood. Stem Cells, 26(8), 2083–2092.
Wojakowski, W., Kucia, M., Zuba-Surma, E., Ratajczak, J., Machalinski, B., Ratajczak, M. Z., et al. (2007). Cardiogenic differentiation of very small embryonic-like cells isolated from adult bone marrow. Journal of the American College of Cardiology. Supplement A, 49(11), 83A.
Ratajczak, M. Z. (2008). Phenotypic and functional characterization of hematopoietic stem cells. Current Opinion in Hematology, 15(4), 293–300.
Shin, D. M., Zuba-Surma, E. K., Wu, W., Ratajczak, J., Wysoczynski, M., Ratajczak, M. Z., et al. (2009). Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia, 23(11), 2042–2051.
Kucia, M., Halasa, M., Wysoczynski, M., Baskiewicz-Masiuk, M., Moldenhawer, S., Zuba-Surma, E., et al. (2007). Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia, 21(2), 297–303.
Kucia, M., Reca, R., Jala, V. R., Dawn, B., Ratajczak, J., & Ratajczak, M. Z. (2005). Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia, 19(7), 1118–1127.
Kucia, M., Dawn, B., Hunt, G., Guo, Y., Wysoczynski, M., Majka, M., et al. (2004). Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circulation Research, 95(12), 1191–1199.
Wojakowski, W., Tendera, M., Kucia, M., Zuba-Surma, E., Paczkowska, E., Ciosek, J., et al. (2009). Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. Journal of the American College of Cardiology, 53(1), 1–9.
Massa, M., Rosti, V., Ferrario, M., Campanelli, R., Ramajoli, I., Rosso, R., et al. (2005). Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood, 105(1), 199–206.
Krankel, N., Katare, R. G., Siragusa, M., Barcelos, L. S., Campagnolo, P., Mangialardi, G., et al. (2008). Role of kinin B2 receptor signaling in the recruitment of circulating progenitor cells with neovascularization potential. Circulation Research, 103(11), 1335–1343.
Wojakowski, W., Tendera, M., Michalowska, A., Majka, M., Kucia, M., Maslankiewicz, K., et al. (2004). Mobilization of CD34/CXCR4+, CD34/CD117+, c-met + stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation, 110(20), 3213–3220.
Kucia, M., Reca, R., Miekus, K., Wanzeck, J., Wojakowski, W., Janowska-Wieczorek, A., et al. (2005). Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells, 23(7), 879–894.
Kucia, M., Wojakowski, W., Reca, R., Machalinski, B., Gozdzik, J., Majka, M., et al. (2006). The migration of bone marrow-derived non-hematopoietic tissue-committed stem cells is regulated in an SDF-1-, HGF-, and LIF-dependent manner. Archivum Immunologiae et Therapiae Experimentalis (Warsz), 54(2), 121–135.
Orlandi, A., Chavakis, E., Seeger, F., Tjwa, M., Zeiher, A. M., & Dimmeler, S. (2010). Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Research in Cardiology, 105(6), 703–712.
Wojakowski, W., Tendera, M., Zebzda, A., Michalowska, A., Majka, M., Kucia, M., et al. (2006). Mobilization of CD34(+), CD117(+), CXCR4(+), c-met(+) stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. European Heart Journal, 27(3), 283–289.
Paczkowska, E., Kucia, M., Koziarska, D., Halasa, M., Safranow, K., Masiuk, M., et al. (2009). Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke, 40(4), 1237–1244.
Wojakowski, W., Tendera, M., Kucia, M., Zuba-Surma, E., Milewski, K., Wallace-Bradley, D., et al. (2010). Cardiomyocyte differentiation of bone marrow-derived Oct-4 + CXCR4 + SSEA-1+ very small embryonic-like stem cells. International Journal of Oncology, 37(2), 237–247.
Dawn, B., Tiwari, S., Kucia, M. J., Zuba-Surma, E. K., Guo, Y., Sanganalmath, S. K., et al. (2008). Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells, 26(6), 1646–1655.
Zuba-Surma, E. K., Guo, Y., Taher, H., Sanganalmath, S. K., Hunt, G., Vincent, R. J., et al. (2010). Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodeling after myocardial infarction. Journal of Cellular and Molecular Medicine, Jul 12. [Epub ahead of print] PMID: 20629987 [PubMed].
Funding
This study is funded by the National Institutes of Health [R01 CA106281-01], European Union structural funds-Innovative Economy Operational Programme grant POIG.01.01.02-00-109/09 “Innovative methods of stem cells applications in medicine” and the Polish Ministry of Science and Higher Education grants 0651/P01/2007/32, 2422/P01/2007/32, and Servier Research grant.
Disclosure
None of the authors have any conflict of interests with regard to the content of this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wojakowski, W., Kucia, M., Liu, R. et al. Circulating Very Small Embryonic-Like Stem Cells in Cardiovascular Disease. J. of Cardiovasc. Trans. Res. 4, 138–144 (2011). https://doi.org/10.1007/s12265-010-9254-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-010-9254-y