Abstract
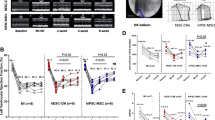
We have investigated the effect of stem cell delivery on the release of hypoxia-inducible factor 1 alpha (HIF-1α) in peripheral circulation and myocardium in experimental myocardial ischemia. Closed-chest, reperfused myocardial infarction (MI) was created in domestic pigs. Porcine mesenchymal stem cells (MSCs) were cultured and delivered (9.8 ± 1.2 × 106) either percutaneously NOGA-guided transendocardially (Group IM) or intracoronary (Group IC) 22 ± 4 days post-MI. Pigs without MSC delivery served as sham control (Group S). Plasma HIF-1α was measured at baseline, immediately post- and at follow-up (FUP; 2 h or 24 h) post-MSC delivery by ELISA kit. Myocardial HIF-1α expression of infarcted, normal myocardium, or border zone was determined by Western blot. Plasma level of HIF-1α increased immediately post-MI (from 278 ± 127 to 631 ± 375 pg/ml, p < 0.05). Cardiac delivery of MSCs elevated the plasma levels of HIF-1α significantly (p < 0.05) in groups IC and IM immediately post-MSC delivery, and returned to baseline level at FUP, without difference between the groups IC and IM. The myocardial tissue HIF-1α expression in the infarcted area was higher in Group IM than in Group IC or S (1,963 ± 586 vs. 1,307 ± 392 vs. 271 ± 110 activity per square millimeter, respectively, p < 0.05), while the border zone contained similarly lower level of HIF-1α, but still significantly higher as compared with Group S. Trend towards increase in myocardial expression of HIF-1α was measured in Group IM at 24 h, in contrast to Group IC. In conclusion, both stem cell delivery modes increase the systemic and myocardial level of HIF-1α. Intramyocardial delivery of MSC seems to trigger the release of angiogenic HIF-1α more effectively than does intracoronary delivery.



Similar content being viewed by others
References
Assmus, B., Schachinger, V., Teupe, C., et al. (2002). Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation, 106, 3009–3017.
Janssens, S., Theunissen, K., Boogaerts, M., & Van de Werf, F. (2006). Bone marrow cell transfer in acute myocardial infarction. Nat Clin Pract Cardiovasc Med, 3(Suppl 1), S69–S72.
Lunde, K., Solheim, S., Aakhus, S., et al. (2006). Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. New England Journal of Medicine, 355, 1199–1209.
Bartunek, J., Croissant, J. D., Wijns, W., et al. (2007). Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factor and repair of the chronically infarcted myocardium. American Journal of Physiology. Heart and Circulatory Physiology, 292, H1095–H1104.
Charwat, S., Gyöngyösi, M., Lang, I., et al. (2008). Role of adult bone marrow stem cells in the repair of ischemic myocardium, current state of the art. Experimental Hematology, 36, 672–680.
Gyöngyösi, M., Lang, I., Dettke, M., et al. (2009). Comparison of early and late combined cardiac application of bone marrow mononuclear stem cells after myocardial infarction. Final results from the MYSTAR prospective randomized study. Nat Clin Pract Cardiovasc Med, 6, 70–81.
Orlic, D., Kajstura, J., Chimenti, S., et al. (2001). Bone marrow cells regenerate infarcted myocardium. Nature, 410, 701–705.
Kocher, A. A., Schuster, M. D., Szabolcs, M. J., et al. (2001). Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nature Medicine, 7, 430–436.
Gyöngyösi, M., Blanco, J., Marian, T., et al. (2008). Serial non-invasive in vivo positron emmission tomographyc (PET) tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circulation Cardiovascular Imaging, 1, 94–103.
Strauer, B., Brehm, M., Zeus, T., et al. (2002). Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation, 106, 1913–1918.
Schachinger, V., Erbs, S., Elsasser, A., et al. (2006). Intracoronary bone marrow derived progenitor cells in acute myocardial infarction. New England Journal of Medicine, 355, 1210–1221.
Orlic, D., Kajstura, J., Chimenti, S., et al. (2001). Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proceedings of the National Academy of Sciences of the United States of America, 98, 10344–10349.
Dimmeler, J., Zeiher, A., & Schneider, M. D. (2005). Unchain my heart, the scientific foundations of cardiac repair. Journal of Clinical Investigation, 115, 572–583.
Wang, G. L. & Semenza, G. L. (1993). General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proceedings of the National Academy of Sciences of the United States of America, 90, 4304–4308.
Wenger, R. H. (2002). Cellular adaptation to hypoxia, O2 sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2 regulated gene expression. FASEB Journal, 16, 1151–1162.
Chan, D. A. & Giaccia, A. J. (2007). Hypoxia, gene expression, and metastasis. Cancer and Metastasis Reviews, 26, 333–339.
Vincent, K. A., Shyu, K. G., Luo, Y., et al. (2000). Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DANN encoding an HIF-1alpha/VP16 hybrid transcription factor. Circulation, 102, 2255–2261.
Wiener, C. M., Booth, G., & Semenza, G. L. (1996). In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochemical and Biophysical Research Communications, 225, 485–488.
Jiang, B., Rue, E., Wang, G. L., Roe, R., & Semenza, G. L. (1996). Dimerization, DANN binding, and transactivation properties of hypoxia-inducible factor 1. Journal of Biological Chemistry, 271, 17771–17778.
Jewell, U. R., Kvietikova, I., Scheid, A., et al. (2001). Induction of HIF-1alpha in response to hypoxia is instantaneous. FASEB Journal, 15, 1312–1314.
Huang, L. E., Gu, J., Schau, M., & Bunn, H. F. (1998). Regulation of hypoxia-inducible factor 1 is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proceedings of the National Academy of Sciences of the United States of America, 95, 7987–7992.
Kallio, P. J., Wilson, W. J., O’Brien, S., Makino, Y., & Poellinger, L. (1999). Regulation of the hypoxia-inducible transcription factor 1 by the ubiquitin-proteasome pathway. Journal of Biological Chemistry, 274, 6519–6525.
Salceda, S. & Caro, J. (1997). Hypoxia-inducible factor 1 (HIF-1) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. Journal of Biological Chemistry, 272, 22642–22647.
Huang, M., Chan, D. A., Jia, F., et al. (2008). Short hairpin RNA interference therapy for ischemic heart disease. Circulation, 118, S226–S233.
Dekel, B., Shezen, E., Even-Tov-Friedman, S., et al. (2006). Transplantation of human hematopoietic stem cells into ischemic and growing kidneys suggests a role in vasculogenesis but not tubulogenesis. Stem Cells, 24, 1185–1193.
Azarnoush, K., Maurel, A., Sebbah, L., et al. (2005). Enhancement of the functional benefits of skeletal myoblast transplantation by means of coadministration of hypoxia-inducible factor 1alpha. Journal of Thoracic and Cardiovascular Surgery, 130(1), 173–179.
Rhoads, R. P., Johnson, R. M., Rathbone, C. R., et al. (2009). Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. American Journal of Physiology. Cell Physiology, 296(6), C1321–C1328.
Ben-Haim, S. A., Osadchy, D., Schuster, I., et al. (1996). Nonfluoroscopic, in vivo navigation and mapping technology. Nature Medicine, 2, 1393–1395.
Gepstein, L., Hayam, G., & Ben-Haim, S. A. (1997). A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart, in vitro and in vivo accuracy results. Circulation, 95, 1611–1622.
Gyöngyösi, M., Sochor, H., Khorsand, A., Gepstein, L., & Glogar, D. (2001). Online myocardial viability assessment in the catheterization laboratory via NOGA electroanatomic mapping. Quantitative comparison with thallium-201 uptake. Circulation, 104, 1005–1011.
Kastrup, J., Jørgensen, E., Rück, A., et al. (2005). Direct intramyocardial plasmid VEGF-A165 gene therapy in patients with stable severe angina pectoris—A randomized double-blind placebo-controlled study—The Euroinject One Trial. Journal of the American College of Cardiology, 45, 982–988.
Ockaili, R., Natarajan, R., Salloum, F., et al. (2005). HIF-1 activation attenuates post-ischemic myocardial injury, a role for heme oxygenase-1 in modulating microvascular chemokine generation. American Journal of Physiology. Heart and Circulatory Physiology, 289, H542–H548.
Xi, L., Taher, M., Yin, C., Salloum, F., & Kukreja, R. C. (2004). Cobalt chloride induces delayed cardiac preconditioning in mice through selective activation of HIF-1alpha and AP-1 and iNOS signaling. American Journal of Physiology. Heart and Circulatory Physiology, 287, H2369–H2375.
Martin, C., Yu, A. Y., Jiang, B. H., et al. (1998). Cardiac hypertrophy in chronically anemic fetal sheep, increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducible factor 1. American Journal of Obstetrics and Gynecology, 178, 527–534.
Bilton, R. L. & Booker, G. W. (2003). The subtle side to hypoxia inducible factor (HIFalpha) regulation. European Journal of Biochemistry, 270, 791–798.
Liu, Y., Cox, S. R., Morita, T., & Kourembanas, S. (1995). Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5= enhancer. Circulation Research, 77, 638–643.
Kim, C. H., Cho, Y. S., Chun, Y. S., et al. (2002). Early expression of myocardial HIF-1alpha in response to mechanical stresses, regulation by stretch-activated channels and the phosphatidylinositol-3-kinase signaling pathway. Circulation Research, 90, E25–E33.
Wang, C., Weihrauch, D., Schwabe, D. A., et al. (2006). Extracellular signal-regulated kinases trigger isoflurane preconditioning concomitant with upregulation of hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression in rats. Anesthesia and Analgesia, 103, 281–288.
Lee, S. H., Wolf, P. L., Escudero, R., et al. (2000). Early expression of angiogenesis factors in acute myocardial ischemia and infarction. New England Journal of Medicine, 342, 626–633.
Pugh, C. W. & Ratcliffe, P. J. (2003). Regulation of angiogenesis by hypoxia, role of the HIF system. Nature Medicine, 9, 677–684.
Chang, E. I., Loh, S. A., Ceradini, D. J., et al. (2007). Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation, 116, 2818–2829.
Loor, G. & Schumacker, P. T. (2008). Role of hypoxia-inducible factor in cell survival during myocardial ischemia–reperfusion. Cell Death and Differentiation, 15, 686–690.
Wang, L. & ChunHua, R. (2009). Mesenchymal stem cells targeting the GVHD. Science in China. Series C, Chemistry, Life Sciences, 52, 603–609.
Aggarwal, S. & Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105, 1815–1822.
Schmid-Brunclik, N., Bürgi-TaboaAntoniou, X., Gassman, M., & Ogunshola, O. O. (2008). Astrocyte responses to injury: VEGF simultaneously modulates cell death and proliferation. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 295, R864–R873.
Acknowledgement
This study was supported by the Ludwig Boltzmann Institute Cluster for Cardiovascular Research and Grant 41-069/2007—Lascor, funded by the Romanian Ministry of Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gyöngyösi, M., Hemetsberger, R., Posa, A. et al. Hypoxia-Inducible Factor 1-Alpha Release After Intracoronary Versus Intramyocardial Stem Cell Therapy in Myocardial Infarction. J. of Cardiovasc. Trans. Res. 3, 114–121 (2010). https://doi.org/10.1007/s12265-009-9154-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-009-9154-1