Abstract
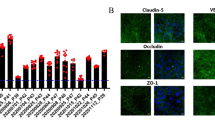
An in vitro blood-brain barrier (BBB) model is critical for enabling rapid screening of the BBB permeability of the drugs targeting on the central nervous system. Though many models have been developed, their reproducibility and renewability remain a challenge. Furthermore, drug transport data from many of the models do not correlate well with the data for in vivo BBB drug transport. Induced-pluripotent stem cell (iPSC) technology provides reproducible cell resources for in vitro BBB modeling. Here, we generated a human in vitro BBB model by differentiating the human iPSC (hiPSC) line GM25256 into brain endothelial-type cells. The model displayed BBB characteristics including tight junction proteins (ZO-1, claudin-5, and occludin) and endothelial markers (von Willebrand factor and Ulex), as well as high trans-endothelial electrical resistance (TEER) (1560 Ω.cm2 ± 230 Ω.cm2) and γ-GTPase activity. Co-culture with primary rat astrocytes significantly increased the TEER of the model (2970 Ω.cm2 to 4185 Ω.cm2). RNAseq analysis confirmed the expression of key BBB-related genes in the hiPSC-derived endothelial cells in comparison with primary human brain microvascular endothelial cells, including P-glycoprotein (Pgp) and breast cancer resistant protein (BCRP). Drug transport assays for nine CNS compounds showed that the permeability of non-Pgp/BCRP and Pgp/BCRP substrates across the model was strongly correlated with rodent in situ brain perfusion data for these compounds (R2 = 0.982 and R2 = 0.9973, respectively), demonstrating the functionality of the drug transporters in the model. Thus, this model may be used to rapidly screen CNS compounds, to predict the in vivo BBB permeability of these compounds and to study the biology of the BBB.






Similar content being viewed by others
References
Goodwin JT, Clark DE. In silico predictions of blood-brain barrier penetration: considerations to “keep in mind”. J Pharmacol Exp Ther 2005, 315: 477–483.
Cho CF, Wolfe JM, Fadzen CM, Calligaris D, Hornburg K, Chiocca EA, et al. Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents. Nat Commun 2017, 8: 15623.
Abbott NJ. Prediction of blood-brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discov Today Technol 2004, 1:407–416.
Liu HF, Dong K, Zhang W, Summerfield SG, Terstappen GC. Prediction of brain-to-blood unbound concentration ratios in CNS drug discovery employing in silico and in vitro model systems. Drug Discov Today 2018, 23: 1357–1372.
Eigenmann DE, Xue G, Kim KS, Moses AV, Hamburger M, Oufir M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10: 33.
Helms HC, Abbott NJ, Burek M, Cecchelli R, Couraud PO, Deli MA, et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab 2016, 36: 862–890.
Weksler BB, Subileau EA, Perrière N, Charneau P, Holloway K, Leveque M, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J 2005, 19: 1872–1874.
Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012, 482: 216–220.
Dolmetsch R, Geschwind DH. The human brain in a dish: the promise of iPSC-derived neurons. Cell 2011, 145: 831–834.
Vatine GD, Al-Ahmad A, Barriga BK, Svendsen S, Salim A, Garcia L, et al. Modeling Psychomotor retardation using iPSCs from MCT8-deficient patients indicates a prominent role for the blood-brain barrier. Cell Stem Cell 2017, 20: 831–843.e5.
Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP, Shusta EV. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep 2014, 4: 4160.
Lim RG, Quan C, Reyes-Ortiz AM, Lutz SE, Kedaigle AJ, Gipson TA, et al. Huntington’s disease iPSC-derived brain microvascular endothelial cells reveal WNT-mediated angiogenic and blood-brain barrier deficits. Cell Rep 2017, 19: 1365–1377.
Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol 2012, 30: 783–791.
Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck MP, et al. Modelling of the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov 2007, 6: 650–661.
Ribecco-Lutkiewicz M, Sodja C, Haukenfrers J, Haqqani AS, Ly D, et al. A novel human induced pluripotent stem cell blood-brain barrier model: Applicability to study antibody-triggered receptor-mediated transcytosis. Sci Rep 2018, 8: 1873.
Katt ME, Xu ZS, Gerecht S, Searson PC. Human brain microvascular endothelial cells derived from the BC1 iPS cell line exhibit a blood-brain barrier phenotype. PLoS One 2016, 11: e0152105.
Skaper SD, Facci L. Culture of neonatal rodent microglia, astrocytes, and oligodendrocytes from the cortex, spinal cord, and cerebellum. Methods Mol Biol 2018, 1727: 49–61.
Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem 1990, 54: 1882–1888.
Rautio J, Humphreys JE, Webster LO, Balakrishnan A, Keogh JP, Kunta JR, et al. In vitro P-glycoprotein inhibition assays for assessment of clinical drug interaction potential of new drug candidates: a recommendation for probe substrates. Drug Metab Dispos 2006, 34: 786–792.
Liu H, Huang L, Li Y, Fu T, Sun X, Zhang YY, et al. Correlation between membrane protein expression levels and transcellular transport activity for breast cancer resistance protein (BCRP). Drug Metabo Dispos 2017, 45: 449–456.
Wilson HK, Faubion MG, Hjortness MK, Palecek SP, Shusta EV. Cryopreservation of brain endothelial cells derived from human induced pluripotent stem cells is enhanced by rho-associated coiled coil-containing kinase inhibition. Tissue Eng Part C Methods 2016, 22: 1085–1094.
El Hafny B, Bourre JM, Roux F. Synergistic stimulation of γ-glutamyl transpeptidase and alkaline phosphatase activities by retinoic acid and astroglial factors in immortalized rat brain microvessel endothelial cells. J Cell Physiol 1996, 167: 451–460.
DeBault LE, Cancilla PA. γ-Glutamyl transpeptidase in isolated brain endothelial cells: induction by glial cells in vitro. Science. 1980, 207: 653–655.
Xiong H, Callaghan D, Bai JY, Jones A, Rasquinha I, Smith C, et al. ABCG2 is up-regulated in Alzheimer’s brain with cerebral amyloid angiopathy and may act as a gatekeeper at the blood-brain barrier for Aβ1-40 peptides. J Neurosci 2009, 29: 5463–5475.
Furihata T, Kawamatsu S, Ito R, Saito K, Suzuki S, Kishida S, et al. Hydrocortisone enhances the barrier properties of HBMEC/ciβ, a brain microvascular endothelial cell line, through mesenchymal-to-endothelial transition-like effects. Fluids Barriers CNS 2015, 12: 7.
Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, et al. Hydrocortisone reinforces the blood-brain properties in a serum free cell culture system. Biochem Biophys Res Commun 1998, 247: 312–315.
Weidenfeller C, Schrot S, Zozulya A, Galla HJ. Murine brain capillary endothelial cells exhibit improved barrier properties under the influence of hydrocortisone. Brain Res 2005, 1053: 162–174.
Mantle JL, Min L, Lee KH. Minimum transendothelial electrical resistance thresholds for the study of small and large molecule drug transport in a human in vitro blood-brain barrier model. Mol Pharm 2016, 13: 4191–4198.
Yi X, Liu M, Luo Q, Zhuo H, Cao H, Wang J, et al. Toxic effects of dimethyl sulfoxide on red blood cells, platelets, and vascular endothelial cells in vitro. FEBS Open Bio 2017, 7:485–494.
de Abreu Costa L, Henrique Fernandes Ottoni M, Dos Santos MG, Meireles AB, Gomes de Almeida V, de Fátima Pereira W, et al. Dimethyl Sulfoxide (DMSO) decreases cell proliferation and TNF-α, IFN-γ, and IL-2 cytokines production in cultures of peripheral blood lymphocytes. Molecules 2017, 22. pii: E1789.
Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, et al. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int 2009, 54: 253–263.
Summerfield SG, Read K, Begley DJ, Obradovic T, Hidalgo IJ, Coggon S, et al. Central nervous system drug disposition: the relationship between in situ brain permeability and brain free fraction. J Pharmacol Exp Ther 2007, 322: 205–213.
Liu H, Li Y, Lu S, Wu Y, Sahi J. Temporal expression of transporters and receptors in a rat primary co-culture blood-brain barrier model. Xenobiotica 2014, 44: 941–951.
Korjamo T, Heikkinen AT, Mönkkönen J. Analysis of unstirred water layer in in vitro permeability experiments. J Pharm Sci 2009, 98: 4469–4479.
Mizee MR, Wooldrik D, Lakeman KA, van het Hof B, Drexhage JA, Geerts D, et al. Retinoic acid induces blood-brain barrier development. J Neurosci 2013, 33: 1660–1671.
Al Ahmad A, Gassmann M, Ogunshola OO. Maintaining blood-brain barrier integrity: pericytes perform better than astrocytes during prolonged oxygen deprivation. J Cell Physiol 2009, 218: 612–622.
Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab 2011, 31: 693–705.
Kikuchi R, de Morais SM, Kalvass JC, In vitro P-glycoprotein efflux ratio can predict the in vivo brain penetration regardless of biopharmaceutics drug disposition classification system class. Drug Metab Dispos 2013. 41: 2012–2017.
Summerfield SG, Zhang Y, Liu H, Examining the uptake of central nervous system drugs and candidates across the blood-brain barrier. J Pharmacol Exp Ther 2016. 358: 294–305.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Sun, X., Liu, H. et al. Development of Human in vitro Brain-blood Barrier Model from Induced Pluripotent Stem Cell-derived Endothelial Cells to Predict the in vivo Permeability of Drugs. Neurosci. Bull. 35, 996–1010 (2019). https://doi.org/10.1007/s12264-019-00384-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-019-00384-7