Abstract
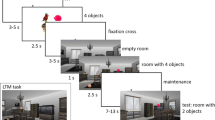
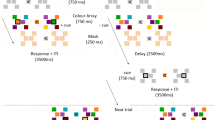
A previous functional magnetic resonance imaging study reported evidence for parallel memory traces in the hippocampus: a controlled match signal detecting matches to internally-generated goal states and an automatic mismatch signal identifying unpredicted perceptual novelty. However, the timing information in this process is unknown. In the current study, facilitated by the high spatial and temporal resolution of intracranial recording from human patients, we confirmed that the left posterior hippocampus played an important role in the goal match enhancement effect, in which combinations of object identity and location were involved. We also found that this effect happened within 520 ms to 735 ms after the probe onset, ~150 ms later than the perceptual mismatch enhancement found bilaterally in both the anterior and posterior hippocampus. More specifically, the latency of the perceptual mismatch enhancement effect of the right hippocampus was positively correlated with the performance accuracy. These results suggested that the hippocampus is crucial in working memory if features binding with location are involved in the task and the goal match enhancement effect happens after perceptual mismatch enhancement, implying the dissociation of different components of working memory at the hippocampus. Moreover, single trial decoding results suggested that the intracranial field potential response in the right hippocampus can classify the match or switch task. This is consistent with the findings that the right hippocampal activity observed during the simulation of the future events may reflect the encoding of the simulation into memory.





Similar content being viewed by others
References
Eichenbaum H. A cortical–hippocampal system for declarative memory. Nature Rev Neurosci 2000, 1: 41–50.
Squire L. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem 2004, 82: 171–177.
Fell J, Axmacher N. The role of phase synchronization in memory processes. Nature Rev Neurosci 2011, 12: 105–118.
Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci 2011, 34: 515–525.
MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum, H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 2011, 71: 737–749.
Kumaran D, Maguire EA. An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol 2006, 4: 1–11.
Maguire EA, Mullally SL. The hippocampus: a manifesto for change. J Exp Psychol 2013, 142: 1180.
Cave CB, Squire LR. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus 1992, 2: 151–163.
Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 2006, 16: 693–700.
Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci 2007, 30: 123.
Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci 2007, 11: 126–135.
Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci 2007, 8: 872–883.
Piekema C, Fernández G, Postma A, Hendriks MP, Wester AJ, Kessels RP. Spatial and non-spatial contextual working memory in patients with diencephalic or hippocampal dysfunction. Brain Res 2007, 1172: 103–109.
Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 2005, 9: 445–453.
Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci 2003, 4: 637–648.
Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci U S A 1998, 95: 906–913.
Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science 1994. 263: 520–522.
Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron 1997, 18: 753–765.
Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol 1997, 78: 1062–1081.
Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. J Neurosci 2008, 28: 116–124.
Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus 2006, 16: 504–520.
Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron 2005, 47: 751–761.
O’Kane G, Insler RZ, Wagner AD. Conceptual and perceptual novelty effects in human medial temporal cortex. Hippocampus 2005, 15: 326–332.
Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron 2008, 60: 378–389.
Piekema C, Kessels RP, Mars RB, Petersson KM, Fernández G. The right hippocampus participates in short-term memory maintenance of object–location associations. Neuroimage 2006, 33: 374–382.
Jutras MJ, Fries P, Buffalo EA. Gamma-band synchronization in the macaque hippocampus and memory formation. J Neurosci 2009, 29: 12521–12531.
Lee ACH, Rudebeck SR. Investigating the interaction between spatial perception and working memory in the human medial temporal lobe. J Cogn Neurosci 2010, 22: 2823–2835.
Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: A high‐resolution fMRI study of the human hippocampus. Hippocampus 2012, 22: 389–398.
Duncan K, Curtis C, Davachi L. Distinct memory signatures in the hippocampus: intentional states distinguish match and mismatch enhancement signals. J Neurosci 2009, 29: 131–139.
Staresina BP, Fell J, Do Lam AT, Axmacher N, Henson RN. Memory signals are temporally dissociated in and across human hippocampus and perirhinal cortex. Nature Neurosci 2012, 15: 1167–1173.
Brainard DH. The psychophysics toolbox. Spat Vis 1997, 10: 433–436.
Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis 1997, 10: 437–442.
Axmacher N, Mormann F, Fernández G, Cohen MX, Elger CE, Fell J, Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci 2007, 27: 7807–7816.
Bishop CM. Neural networks for pattern recognition. Oxford University Press, 1995.
Addis DR, Cheng T, P Roberts R, Schacter DL. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus 2011, 21: 1045–1052.
Nahum L, Gabriel D, Spinelli L, Momjian S, Seeck M, Michel CM, et al. Rapid consolidation and the human hippocampus: Intracranial recordings confirm surface EEG. Hippocampus 2011, 21: 689–693.
James C, Morand S, Barcellona‐Lehmann S, Michel CM, Schnider A. Neural transition from short‐to long‐term memory and the medial temporal lobe: A human evoked‐potential study. Hippocampus 2009, 19: 371–378.
Ludowig E, Bien CG, Elger CE, Rosburg T. Two P300 generators in the hippocampal formation. Hippocampus 2010, 20: 186–195.
Guo C, Lawson AL, Zhang Q, Jiang Y. Brain potentials distinguish new and studied objects during working memory. Hum Brain Mapp 2008, 29: 441–452.
Tudesco Ide S, Vaz LJ, Mantoan MAS, Belzunces E, Noffs MH, Caboclo LO, et al. Assessment of working memory in patients with mesial temporal lobe epilepsy associated with unilateral hippocampal sclerosis. Epilepsy Behav 2010, 18: 223–228.
Finke C, Braun M, Ostendorf F, Lehmann TN, Hoffmann KT, Kopp U, et al. The human hippocampal formation mediates short-term memory of colour–location associations. Neuropsychologia 2008, 46: 614–623.
Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci 2008, 9: 182–194.
Acknowledgements
We thank the patients for their cooperation in this study. We also thank Gabriel Kreiman, Jedediah Singer and Kun Hu for their assistance and support. Many thanks to the doctors and nurses in Beijing Functional Neurosurgery Institute for their cooperation, especially Xueyuan Wang, Xi Zhang, Cuiping Xu, and Chang Liu. We also acknowledge Fenghuachangtai Company for providing the device for ECoG recording. This work was supported by grants from the Ministry of Science and Technology of China (2015CB351701 and 2012CB825500), the National Natural Science Foundation of China (91132302), and the Chinese Academy of Sciences (XDB2010001 and XDB2050001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ni, B., Wu, R., Yu, T. et al. Role of the Hippocampus in Distinct Memory Traces: Timing of Match and Mismatch Enhancement Revealed by Intracranial Recording. Neurosci. Bull. 33, 664–674 (2017). https://doi.org/10.1007/s12264-017-0172-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-017-0172-8