Abstract
Objective
Nitric oxide (NO) was speculated to play an important role in the pathophysiology of cerebral ischemia. Minocycline, a tetracycline derivative, reduced inflammation and protected against cerebral ischemia. To study the neuroprotection mechanism of minocycline for vascular dementia, the influences of minocycline on expressions of inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) were observed in the brains of Wistar rats.
Methods
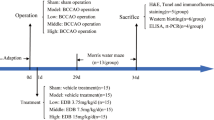
The vascular dementia rat model was established by permanent bilateral common carotid arteries occlusion (BCCAO). Wistar rats were divideded into 3 groups randomly: sham-operation group (S group), vascular dementia model group (M group), and minocycline treatment group (MT group). The behaviour was tested with Morris water maze and open-field task. Expressions of iNOS and eNOS were measured by immunohistochemistry and reverse transcriptase-polymerase chain reaction (RT-PCR). The optical density value was measured by imaging analysis. Percentage of positive cells with iNOS and eNOS expression was analyzed with optical microscope.
Results
Minocycline attenuated cognitive impairment. Inducible NOS was significantly down-regulated in MT group, compared with that in M group (P < 0.01), while eNOS was significantly up-regulated, compared with that in M group (P < 0.01). The expressions of iNOS and eNOS in M and MT groups were higher than those in S group (P < 0.01).
Conclusion
Minocycline can down-regulate the expression of iNOS and up-regulate the expression of eNOS in vascular dementia, which restrains apoptosis and oxidative stress to protect neural function.
摘要
目的
观察美满霉素(minocycline)对血맜性痴呆大鼠学习记忆功能和脑组织内皮型一氧化氮合酶(endothelial nitric oxide synthase, eNOS)、 诱导型一氧化氮合酶(inducible nitric oxide synthase, iNOS)表达的影响, 探讨美满霉素对血맜性痴呆的脑保护作用的机制。
方法
Wistar大鼠随机分为假手术组(S 组)、 痴呆模型组(M 组)、 美满霉素治疗组(MT组)。 RT-PCR和免疫组织化学法检测大鼠脑组织eNOS、iNOS的表达, 行为学检测大鼠学习记忆功能的改变。
结果
M组与S组行为学检查显示, M组大鼠有显著学习记忆障碍(P < 0.01), MT组与M组比较行为学检测结果显示, MT 组大鼠学习记忆障碍有显著改善(P < 0.01)。 MT组iNOS表达较M组降低(P < 0.01), MT组eNOS表达较M组增高(P < 0.05); MT组eNOS、 iNOS表达较S组增高(P < 0.01); M组eNOS、 iNOS表达较S组显著增高(P < 0.01)。
结论
美满霉素能降低血맜性痴呆大鼠脑组织iNOS表达, 增强eNOS 表达, 抑制氧化应激反应, 发挥脑保护作用。
Similar content being viewed by others
References
Morimoto N, Shimazawa M, Yamashima T, Nagai H, Hara H. Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Brain Res 2005, 1044: 8–15.
Lin S, Zhang Y, Dodel R, Farlow MR, Paul SM, Du Y. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett 2001, 315: 61–64.
Yrjänheikki J, Tikka T, Keinänen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A 1999, 96: 13496–13500.
Yu CY, Cai ZY. Effect of minocycline on expression of MMP-2 and MMP-9 in rats with focal cerebral ischemic-reperfusion. J Guizhou Med China 2006, 30: 983–985. (Chinese, English abstract)
Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci 2004, 24: 2182–2190.
Hewlett KA, Corbett D. Delayed minocycline treatment reduces long-term functional deficits and histological injury in a rodent model of focal ischemia. Neuroscience 2006, 141: 27–33.
Vannucchi MG, Bizzoco E, Corsani L, Gianfriddo M, Pedata F, Faussone-Pellegrini MS. Relationships between neurons expressing neuronal nitric oxide synthase, degree of microglia activation and animal survival. A study in the rat cortex after transient ischemia. Brain Res 2007, 1132: 218–227.
Pluta RM, Rak R, Wink DA, Woodward JJ, Khaldi A, Oldfield EH, et al. Effects of nitric oxide on reactive oxygen species production and infarction size after brain reperfusion injury. Neurosurgery, 2001, 48: 884–893.
Mishra OP, Mishra R, Ashraf QM, Delivoria-Papadopoulos M. Nitric oxide-mediated mechanism of neuronal nitric oxide synthase and inducible nitric oxide synthase expression during hypoxia in the cerebral cortex of newborn piglets. Neuroscience 2006, 140: 857–863.
Han F, Shirasaki Y, Fukunaga K. Microsphere embolism-induced endothelial nitric oxide synthase expression mediates disruption of the blood-brain barrier in rat brain. J Neurochem 2006, 99: 97–106.
Chi OZ, Hunter C, Liu X, Weiss HR. Effects of VEGF and nitric oxide synthase inhibition on blood-brain barrier disruption in the ischemic and non-ischemic cerebral cortex. Neurol Res 2005, 27: 864–868.
Weng YC, Kriz J. Differential neuroprotective effects of a minocycline-based drug cocktail in transient and permanent focal cerebral ischemia. Exp Neurol 2007, 204: 433–442.
Rosenberg GA, Estrada EY, Mobashery S. Effect of synthetic matrix metalloproteinase inhibitors on lipopolysaccharide-induced blood-brain barrier opening in rodents: Differences in response based on strains and solvents. Brain Res 2007, 1133: 186–192.
Xu L, Fagan SC, Waller JL, Edwards D, Borlongan CV, Zheng J, et al. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol 2004, 4: 7.
Pattison LR, Kotter MR, Fraga D, Bonelli RM. Apoptotic cascades as possible targets for inhibiting cell death in Huntington’s disease. J Neurol 2006, 253: 1137–1142.
Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Kim HS, et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology 2007, 32: 2393–2404.
Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, et al. Minocycline inhibits cytochrome C release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002, 417: 74–78.
Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, et al. Minocycline inhibits caspase-independent and-dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci U S A 2003, 100: 10483–10487.
Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci 2002, 22: 1763–1771.
Chu LS, Fang SH, Zhou Y, Yu GL, Wang ML, Zhang WP, et al. Minocycline inhibits 5-lipoxygenase activation and brain inflammation after focal cerebral ischemia in rats. Acta Pharmacol Sin 2007, 28: 763–772.
Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke 2007, 38: 146–152.
Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke 2006, 37: 1087–1093.
Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma 2000, 17: 871–890.
Keynes RG, Garthwaite J. Nitric oxide and its role in ischemic brain injury. Curr Mol Med 2004, 4: 179–191.
Yap YW, Whiteman M, Cheung NS. Chlorinative stress: an under appreciated mediator of neurodegeneration? Cell Signal 2007, 19: 219–228.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, ZY., Yan, Y., Sun, SQ. et al. Minocycline attenuates cognitive impairment and restrains oxidative stress in the hippocampus of rats with chronic cerebral hypoperfusion. Neurosci. Bull. 24, 305–313 (2008). https://doi.org/10.1007/s12264-008-0324-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-008-0324-y