Abstract
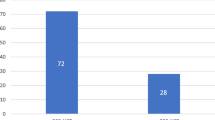
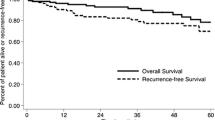
Data from India regarding the disease spectrum and surgical results of neuroendocrine tumours (GEPNETs) are sparse. Tempered surgical radicality in a high-volume oncology centre, conforming to existing guidelines, may further our understanding of tumour characteristics and behavioural patterns of nonfunctional GEPNETs. Surgical outcomes of patients with histopathologically confirmed GEPNETs from January 2003 to December 2013 were analyzed from a prospectively maintained database. Tumour grade, organ of primary tumour, perioperative factors, quality/radicality of resection and presence of metastatic disease were correlated with perioperative outcomes, overall survival and disease-free survival. Ninety of the 101 operated patients had nonfunctional tumours. These comprised radical resections (n = 69), organ-preserving procedures (n = 16) and inoperable tumours (n = 5). The primary tumour sites were pancreatic in 48 patients and gastroenteric in 42 patients. The overall perioperative morbidity and mortality rates were 30 and 3 %, respectively. Fifteen patients harboured metastatic disease at presentation. At a median follow-up of 22 months, 18 patients had residual disease, 7 developed recurrences and 10 patients died. The estimated actuarial 5-year overall survival was 81.6 %, and disease-free survival was 67.2 %. Tumour grade and organ of origin (pancreatic vs. gastroenteric) did not influence long-term survival (p = 0.315 and p = 0.624, respectively), but presence of metastatic disease at presentation significantly affected long-term survival (p = 0.009). Nonfunctional pancreatic/duodenal neuroendocrine tumours (NETs) accounted for 76 % of surgical resections at our centre with the minority being other resections. In selected patients with nonfunctional NETs, organ-preserving surgery may provide equivalent long-term survival with decreased operative morbidity. Although tumour grade is considered to be an important prognostic factor, the presence of metastatic disease at presentation also determines long-term survival. The referral bias suggests the need for greater awareness given the favourable long-term outcomes of these tumours. There is a need to correct this referral bias by increasing the awareness of GEPNETs in India.


Similar content being viewed by others
References
Ramage JK, Ahmed A, Ardill J et al (2012) Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 61:6–32
Hauso O, Gustafsson BI, Kidd M et al (2008) Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer 113:2655–2664
Modlin IM, Oberg K, Chung DC et al (2008) Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9:61–72
Yao JC, Hassan M, Phan A et al (2008) One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 26:3063–3072
Rindi G, Arnold R, Bosman F, et al. (2010) Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: WHO classification of tumors of the digestive system. Edited by Bosman F, Hruban R, Theise N. Lyon: IARC Press p. 13-14
Pape UF, Jann H, Muller-Nordhorn J et al (2008) Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 113:256–265
Ito H, Abramson M, Ito K et al (2010) Surgery and staging of pancreatic neuroendocrine tumors: a 14 years experience. J Gastrointest Surg 14:891–898
Norton JA, Kivlen M, Li M et al (2003) Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg 138:859–866
Fischer L, Kleeff J, Esposito I et al (2008) Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg 95:627–635
Birnbaum DJ, Turrini O, Vigano L et al (2014) Surgical management of advanced pancreatic neuroendocrine tumors: short-term and long-term results from an international multi-institutional study. Ann Surg Oncol 12:234–239
Song KB, Kim SC, Kim JH et al (2014) Prognostic factors in 151 patients with surgically resected non-functioning pancreatic neuroendocrine tumours. ANZ J Surg 123:230–238
Hu HK, Ke NW, Li A et al (2015) Clinical characteristics and prognostic factors of gastroenteropancreatic neuroendocrine tumors: a single center experience in China. Hepatogastroenterology 62:178–183
Amarapurkar DN, Juneja MP, Patel ND et al (2010) A retrospective clinico-pathological analysis of neuroendocrine tumors of the gastrointestinal tract. Trop Gastroenterol 31:101–104
Bhatia P, Srinivasan R, Rajwanshi A et al (2008) 5 years review and reappraisal of ultrasound-guided percutaneous transabdominal fine needle aspiration of pancreatic lesions. Acta Cytol 52:523–529
Sali PA, Shah R, Jagannath P (2014) Experience with the technique of pancreas-sparing distal duodenectomy. Indian J Gastroenterol 33:63–66
Turaga KK, Kvols LK (2011) Recent progress in the understanding, diagnosis, and treatment of gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin 61:113–132
Shrikhande SV, Sirohi B, Goel M et al (2013) Pancreatic neuroendocrine tumors. Indian J Gastroenterol 32:3–17
Wente MN, Bassi C, Dervenis C et al (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142:761–768
Bassi C, Dervenis C, Butturini G et al (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13
Mangram AJ, Horan TC, Pearson ML et al (1999) Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20:250–278, quiz 279–280
Rinke A, Muller HH, Schade-Brittinger C et al (2009) Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 27:4656–4663
Kwekkeboom DJ, de Herder WW, Kam BL (2008) Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 26:2124–2130
Schurr PG, Strate T, Rese K et al (2007) Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg 245:273–281
Nguyen SQ, Angel LP, Divino CM et al (2007) Surgery in malignant pancreatic neuroendocrine tumors. J Surg Oncol 96:397–403
Thirlby RC (1995) Management of patients with gastric carcinoid tumors. Gastroenterology 108:296–297
Hellman P, Lundstrom T, Ohrvall U et al (2002) Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg 26:991–997
Bernick PE, Klimstra DS, Shia J et al (2004) Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum 47:163–169
Falconi M, Zerbi A, Crippa S et al (2010) Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol 17:1621–1627
Jagannath P, Chhabra D, Shrikhande S et al (2012) Surgical treatment of liver metastases in neuroendocrine neoplasms. Int J Hepatol 112:666–672
Shrikhande SV, Barreto SG, Somashekhar BA et al (2013) Evolution of pancreatoduodenectomy in a tertiary cancer center in India: improved results from service reconfiguration. Pancreatology 13:63–71
Acknowledgments
Dr. Clarence J. Samuel for statistical assistance
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Financial Disclosure
None.
Rights and permissions
About this article
Cite this article
Gaikwad, V., Patkar, S., Barreto, S.G. et al. Outcomes of 101 Consecutive Surgical Resections of Gastroenteropancreatic Neuroendocrine Tumours (GEPNETs) at Tata Memorial Hospital: a Referral Bias for Nonfunctional Duodenopancreatic Tumours and the Need for Greater Awareness of GEPNETs as a Distinct Entity. Indian J Surg 79, 226–233 (2017). https://doi.org/10.1007/s12262-016-1453-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-016-1453-6