Abstract
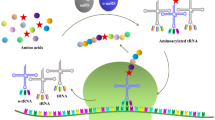
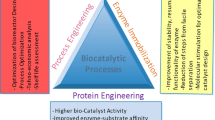
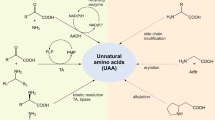
The development of new enzyme engineering technologies has been actively pursued as the industrial use of biocatalysts is rapidly increasing. Traditional enzyme engineering has been limited to changing the functional properties of enzymes by replacing one amino acid with the other 19 natural amino acids. However, the incorporation of unnatural amino acids (UAAs) has been exploited to manipulate efficient enzymes for biocatalysis. This has been an effective enzyme engineering technique by complementing and extending the limits of traditional enzymatic functional changes. This review paper describes the basic functions of the new functional groups of UAAs used in enzyme engineering and the utilization of UAAs in the formation of chemical bonds in the proteins. The recent developments of UAA-mediated enzymology and its applicability in industry, pharmaceutical and other research areas to overcome the limitations of existing enzymes is also emphasized.
Similar content being viewed by others
References
Ravikumar, Y., S. P. Nadarajan, T. H. Yoo, C. S. Lee, and H. Yun (2015) Incorporating unnatural amino acids to engineer biocatalysts for industrial bioprocess applications. Biotechnol. J. 10: 1862–1876.
Aissaoui, N., J. Landoulsi, L. Bergaoui, S. Boujday, and J.-F. Lambert (2013) Catalytic activity and thermostability of enzymes immobilized on silanized surface: Influence of the crosslinking agent. Enzyme Microb. Technol. 52: 336–343.
Baslé, E., N. Joubert, and M. Pucheault (2010) Protein chemical modification on endogenous amino acids. Chem Biol. 17: 213–227.
Drahl, C., B. F. Cravatt, and E. J. Sorensen (2005) Protein-reactive natural products. Angew. Chem. Int. Ed. Engl. 44: 5788–5809.
Kaiser, E. T. and D. S. Lawrence (1984) Chemical mutation of enzyme active sites. Science. 226: 505–511.
Ravikumar, Y., S. P. Nadarajan, T. H. Yoo, C. S. Lee, and H. Yun (2015) Unnatural amino acid mutagenesis-based enzyme engineering. Trends Biotechnol. 33: 462–470.
Zheng, S. and I. Kwon (2012) Manipulation of enzyme properties by noncanonical amino acid incorporation. Biotechnol. J. 7: 47–60.
Link, A. J., M. K. S. Vink, N. J. Agard, J. A. Prescher, C. R. Bertozzi, and D. A. Tirrell (2006) Discovery of aminoacyl-tRNA synthetase activity through cell-surface display of noncanonical amino acids. Proc. Natl. Acad. Sci. USA. 103: 10180–10185.
Link, A. J. and D. A. Tirrell (2005) Reassignment of sense codons in vivo. Methods. 36: 291–298.
Minks, C., S. Alefelder, L. Moroder, R. Huber, and N. Budisa (2000) Towards new protein engineering: in vivo building and folding of protein shuttles for drug delivery and targeting by the selective pressure incorporation (SPI) method. Tetrahedron. 56: 9431–9442.
Rajesh Mehta, K., C. Y. Yang, and J. K. Montclare (2011) Modulating substrate specificity of histone acetyltransferase with unnatural amino acids. Mol. BioSyst. 7: 3050–3055.
Merkel, L., M. Schauer, G. Antranikian, and N. Budisa (2010) Parallel incorporation of different fluorinated amino acids: on the way to ‘teflon’ proteins. Chembiochem. 11: 1505–1507.
Soumillion, P. and J. Fastrez (1998) Incorporation of 1,2,4-triazole-3-alanine into a mutant of phage lambda lysozyme containing a single histidine. Protein Eng. Des. Sel. 11: 213–217.
Schlesinger, S. (1968) The effect of amino acid analogues on alkaline phosphatase formation in Escherichia coli K-12 II. Replacement of tryptophan by azatryptophan and by tryptazan. J. Biol. Chem. 243: 3877–3883.
Wang, L., J. Xie, and P. G. Schultz (2006) Expanding the genetic code. Ann. Rev. Biophys. Biomol. Struct. 35: 225–249.
Noren, C. J., S. J. Anthony-Cahill, M. C. Griffith, and P. G. Schultz (1989) A general method for site-specific incorporation of unnatural amino acids into proteins. Science. 244: 182–188.
Bain, J. D., E. S. Diala, C. G. Glabe, D. A. Wacker, M. H. Lyttle, T. A. Dix, and A. R. Chamberlin (1991) Site-specific incorporation of non-natural residues during in vitro protein biosynthesis with semi-synthetic aminoacyl-tRNAs. Biochemistry. 30: 5411–5421.
Furter, R. (1998) Expansion of the genetic code: site-directed p-fluoro-phenylalanine incorporation in Escherichia coli. Protein Sci. 7: 419–426.
Wang, L., A. Brock, B. Herberich, and P. G. Schultz (2001) Expanding the genetic code of Escherichia coli. Science. 292: 498–500.
Cadwell, R. C. and G. F. Joyce (1992) Randomization of genes by PCR mutagenesis. Genome Res. 2: 28–33.
Abou-Nader, M. and M. J. Benedik (2010) Rapid generation of random mutant libraries. Bioeng Bugs. 1: 337–340.
Vogel, H. J., D. J. Schibli, W. Jing, E. M. Lohmeier-Vogel, R. F. Epand, and R. M. Epand (2002) Towards a structure-function analysis of bovine lactoferricin and related tryptophan- and arginine-containing peptides. Biochem. Cell Biol. 80: 49–63.
Liu, C. C. and P. G. Schultz (2010) Adding new chemistries to the genetic code. Annu. Rev. Biochem. 79: 413–444.
Kirshenbaum, K., I. S. Carrico, and D. A. Tirrell (2002) Biosynthesis of proteins incorporating a versatile set of phenylalanine analogues. Chembiochem. 3: 235–237.
Tang, Y. and D. A. Tirrell (2002) Attenuation of the editing activity of the Escherichia coli leucyl-tRNA synthetase allows incorporation of novel amino acids into proteins in vivo. Biochemistry. 41: 10635–10645.
Wang, P., Y. Tang, and D. A. Tirrell (2003) Incorporation of trifluoroisoleucine into proteins in vivo. J. Am. Chem. Soc. 125: 6900–6906.
Zhao, S., J. Dai, M. Z. Hu, C. Liu, R. S. Meng, X. Liu, C. Wang, and T. Luo (2016) Photo-induced coupling reactions of tetrazoles with carboxylic acids in aqueous solution: application in protein labelling. Chem. Commun. 52: 4702–4705.
Tanaka, Y., M. R. Bond, and J. J. Kohler (2008) Photocrosslinkers illuminate interactions in living cells. Mol. BioSyst. 4: 473–480.
Sato, S., S. Mimasu, A. Sato, N. Hino, K. Sakamoto, T. Umehara, and S. Yokoyama (2011) Crystallographic study of a site-specifically cross-linked protein complex with a genetically incorporated photoreactive amino acid. Biochemistry. 50: 250–257.
Liu, T., Y. Wang, X. Luo, J. Li, S. A. Reed, H. Xiao, T. S. Young, and P. G. Schultz (2016) Enhancing protein stability with extended disulfide bonds. Proc. Natl. Acad. Sci. USA. 113: 5910–5915.
Kadokura, H., F. Katzen, and J. Beckwith (2003) Protein disulfide bond formation in prokaryotes. Ann. Rev. Biochem. 72: 111–135.
Cappadocia, L. and C. D. Lima (2018) Ubiquitin-like protein conjugation: structures, chemistry, and mechanism. Chem. Rev. 118: 889–918.
Sevier, C. S. and C. A. Kaiser (2002) Formation and transfer of disulphide bonds in living cells. Nature Rev. Mol. Cell Biol. 3: 836–847.
Mayer, C., D. G. Gillingham, T. R. Ward, and D. Hilvert (2011) An artificial metalloenzyme for olefin metathesis. Chem. Commun. 47: 12068–12070.
Kuang, H. and M. D. Distefano (1998) Catalytic enantioselective reductive amination in a host—guest system based on a protein cavity. J. Am. Chem. Soc. 120: 1072–1073.
Dombkowski, A. A., K. Z. Sultana, and D. B. Craig (2014) Protein disulfide engineering. FEBS Lett. 588: 206–212.
Wart, H. E. V., A. Lewis, H. A. Scheraga, and F. D. Saeva (1973) Disulfide bond dihedral angles from raman spectroscopy. Proc. Natl. Acad. Sci. USA. 70: 2619–2623.
Chin, J. W., S. W. Santoro, A. B. Martin, D. S. King, L. Wang, and P. G. Schultz (2002) Addition of p-azido-L-phenylalanine to the genetic code of Escherichia coli. J. Am. Chem. Soc. 124: 9026–9027.
Tippmann, E. M., W. Liu, D. Summerer, A. V. Mack, and P. G. Schultz (2007) A genetically encoded diazirine photocrosslinker in Escherichia coli. Chembiochem. 8: 2210–2214.
Ai, H. W., W. Shen, A. Sagi, P. R. Chen, and P. G. Schultz (2011) Probing Protein—protein interactions with a genetically encoded photo-crosslinking amino acid. Chembiochem. 12: 1854–1857.
Chou, C., R. Uprety, L. Davis, J. W. Chin, and A. Deiters (2011) Genetically encoding an aliphatic diazirine for protein photocrosslinking. Chem. Sci. 2: 480–483.
Zhang, M., S. Lin, X. Song, J. Liu, Y. Q. Fu, X. Ge, X. Fu, Z. Chang, and P. R. Chen (2011) A genetically incorporated crosslinker reveals chaperone cooperation in acid resistance. Nat. Chem. Biol. 7: 671–677.
Lin, S., Z. J. Zhang, H. Xu, L. Li, S. Chen, J. Li, Z. Hao, and P. R. Chen (2011) Site-specific incorporation of photo-cross-linker and bio-orthogonal amino acids into enteric bacterial pathogens. J. Am. Chem. Soc. 133: 20581–20587.
Chin, J. W., A. B. Martin, D. S. King, L. Wang, and P. G. Schultz (2002) Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proc. Natl. Acad. Sci. USA. 99: 11020–11024.
Hino, N., M. Oyama, A. Sato, T. Mukai, F. Iraha, A. Hayashi, H. Kozuka-Hata, T. Yamamoto, S. Yokoyama and K. Sakamoto (2011) Genetic incorporation of a photocrosslinkable amino acid reveals novel protein complexes with GRB2 in mammalian cells. J. Mol. Biol. 406: 343–353.
Coin, I., V. Katritch, T. Sun, Z. Xiang, F. Y. Siu, M. Beyermann, R. C. Stevens, and L. Wang (2013) Genetically encoded chemical probes in cells reveal the binding path of urocortin-I to CRF class B GPCR. Cell. 155: 1258–1269.
Ray-Saha, S., T. Huber, and T. P. Sakmar (2014) Antibody epitopes on g protein-coupled receptors mapped with genetically encoded photoactivatable cross-linkers. Biochemistry. 53: 1302–1310.
Dugan, A., C. Y. Majmudar, R. Pricer, S. Niessen, J. K. Lancia, H. Y. Fung, B. F. Cravatt, and A. K. Mapp (2016) Discovery of enzymatic targets of transcriptional activators via in vivo covalent chemical capture. J. Am. Chem. Soc. 138: 12629–12635.
Wilkins, B. J., N. A. Rall, Y. Ostwal, T. Kruitwagen, K. Hiragami-Hamada, M. Winkler, Y. Barral, W. Fischle, and H. Neumann (2014) A cascade of histone modifications induces chromatin condensation in mitosis. Science. 343: 77–80.
Li, J. C., T. Liu, Y. Wang, A. P. Mehta, and P. G. Schultz (2018) Enhancing protein stability with genetically encoded noncanonical amino acids. J. Am. Chem. Soc. 140: 15997–16000.
Banerjee, D., A. P. Liu, N. R. Voss, S. L. Schmid, and M. G. Finn (2010) Multivalent display and receptor-mediated endocytosis of transferrin on virus-like particles. ChemBioChem. 11: 1273–1279.
Rostovtsev, V. V., L. G. Green, V. V. Fokin, and K. B. Sharpless (2002) A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective ‘ligation’ of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 41: 2596–2599.
Steinmetz, N. F., V. Hong, E. D. Spoerke, P. Lu, K. Breitenkamp, M. G. Finn, and M. Manchester (2009) Buckyballs meet viral nanoparticles: candidates for biomedicine. J. Am. Chem. Soc. 131: 17093–17095.
Xuan, W., D. Collins, M. Koh, S. Shao, A. Yao, H. Xiao, P. Garner, and P. G. Schultz (2018) Site-specific incorporation of a thioester containing amino acid into proteins. ACS Chem. Biol. 13: 578–581.
Xiang, Z., V. K. Lacey, H. Ren, J. Xu, D. J. Burban, P. A. Jennings, and L. Wang (2014) Proximity-enabled protein crosslinking through genetically encoding haloalkane unnatural amino acids. Angew. Chem. Int. Ed. Engl. 53: 2190–2193.
Chen, X. H., Z. Xiang, Y. S. Hu, V. K. Lacey, H. Cang, and L. Wang (2014) Genetically encoding an electrophilic amino acid for protein stapling and covalent binding to native receptors. ACS Chem. Biol. 9: 1956–1961.
Xuan, W., S. Shao, and P. G. Schultz (2017) Protein crosslinking by genetically encoded nncanonical amino acids with reactive aryl carbamate side chains. Angew. Chem. Int. Ed. Engl. 56: 5096–5100.
Wang, N., B. Yang, C. Fu, H. Zhu, F. Zheng, T. Kobayashi, J. Liu, S. Li, C. Ma, P. G. Wang, Q. Wang, and L. Wang (2018) Genetically encoding fluorosulfate-l-tyrosine to react with lysine, histidine, and tyrosine via SuFEx in proteins in vivo. J. Am. Chem. Soc. 140: 4995–4999.
Xuan, W., J. Li, X. Luo, and P. G. Schultz (2016) Genetic incorporation of a reactive isothiocyanate group into proteins. Angew. Chem. Int. Ed. Engl. 55: 10065–10068.
Kim, S., W. Ko, B. H. Sung, S. C. Kim, and H. S. Lee (2016) Direct protein—protein conjugation by genetically introducing bioorthogonal functional groups into proteins. Bioorg. Med. Chem. 24: 5816–5822.
Chen, X., J. L. Zaro, and W. C. Shen (2013) Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 65: 1357–1369.
Bai, Y. and W. C. Shen (2006) Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferrin fusion protein by spacer optimization. Pharm. Res. 23: 2116–2121.
McCormick, A. L., M. S. Thomas, and A. W. Heath (2001) Immunization with an interferon-gamma-gp120 fusion protein induces enhanced immune responses to human immunodeficiency virus gp120. J. Infect. Dis. 184: 1423–1430
Bergeron, L. M., L. Gomez, T. A. Whitehead, and D. S. Clark (2009) Self-renaturing enzymes: design of an enzyme-chaperone chimera as a new approach to enzyme stabilization. Biotechnol. Bioeng. 102: 1316–1322.
Bartlett, G. J., C. T. Porter, N. Borkakoti, and J. M. Thornton (2002) Analysis of catalytic residues in enzyme active sites. J. Mol. Biol. 324: 105–121.
McCall, K. A., C. Huang, and C. A. Fierke (2000) Function and mechanism of zinc metalloenzymes. J. Nutr. 130: 1437S–1446S.
Vallee, B. L. and D. S. Auld (1990) Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 29: 5647–5659.
Oikawa, A. (1959) The role of calcium in Taka-amylase A: II. The exchange reaction of calcium. J. Biochem. 46: 463–473.
Toda, H., I. Kato, and K. Narita (1968) Correlation of the masked sulfhydryl group with the essential calcium in Takaamylase A. J. Biochem. 63: 295–301.
Lu, Y., N. Yeung, N. Sieracki, and N. M. Marshall (2009) Design of functional metalloproteins. Nature. 460: 855–862.
Waldron, K. J., J. C. Rutherford, D. Ford, and N. J. Robinson (2009) Metalloproteins and metal sensing. Nature. 460: 823–830
Rosati, F. and G. Roelfes (2010) Artificial metalloenzymes. ChemCatChem. 2: 916–927.
Reetz, M. T., M. Rentzsch, A. Pletsch, A. Taglieber, F. Hollmann, R. J. Mondière, N. Dickmann, B. Höcker, S. Cerrone, M. C. Haeger, and R. Sterner (2008) A robust protein host for anchoring chelating ligands and organocatalysts. ChemBioChem. 9: 552–564.
Matsuo, T. and S. Hirota (2014) Artificial enzymes with protein scaffolds: structural design and modification. Bioorg. Med. Chem. 22: 5638–5656.
Drienovska, I., A. Rioz-Martínez, A. Draksharapu, and G. Roelfes (2015) Novel artificial metalloenzymes by in vivo incorporation of metal-binding unnatural amino acids. Chem. Sci. 6: 770–776.
Lee, H. S. and P. G. Schultz (2008) Biosynthesis of a site-specific DNA cleaving protein. J. Am. Chem. Soc. 130: 13194–13195.
Yang, H., P. Srivastava, C. Zhang, and J. C. Lewis (2014) A general method for artificial metalloenzyme formation through strain-promoted azide.alkyne cycloaddition. ChemBioChem. 15: 223–227.
Glazer, A. N. (1970) Specific chemical modification of proteins. Annu. Rev. Biochem. 39: 101–130.
Wong, L. S., F. Khan, and J. Micklefield (2009) Selective covalent protein immobilization: strategies and applications. Chem. Rev. 109: 4025–4053.
Umeda, A., G. N. Thibodeaux, J. Zhu, Y. A. Lee, and Z. J. Zhang (2009) Site-specific protein cross-linking with genetically incorporated 3,4-dihydroxy-L-phenylalanine. ChemBioChem. 10: 1302–1304.
Yang, B., N. Ayyadurai, H. Yun, Y. S. Choi, B. H. Hwang, J. Huang, Q. Lu, H. Zeng, and H. J. Cha (2014) In vivo residuespecific dopa-incorporated engineered mussel bioglue with enhanced adhesion and water resistance. Angew. Chem. Int. Ed. Engl. 53: 13360–13364.
Ayyadurai, N., N. S. Prabhu, K. Deepankumar, Y. J. Jang, N. Chitrapriya, E. Song, N. Lee, S. K. Kim, B. G. Kim, N. Soundrarajan, S. Lee, H. J. Cha, N. Budisa, and H. Yun (2011) Bioconjugation of L-3,4-dihydroxyphenylalanine containing protein with a polysaccharide. Bioconjug Chem. 22: 551–555.
Deepankumar, K., S. P. Nadarajan, S. Mathew, S. G. Lee, T. H. Yoo, E. Y. Hong, B. G. Kim, and H. Yun (2015) Engineering transaminase for stability enhancement and site-specific immobilization through multiple noncanonical amino acids incorporation. ChemCatChem. 7: 417–421.
Lim, S. I., Y. Mizuta, A. Takasu, Y. H. Kim, and I. Kwon (2014) Site-specific bioconjugation of a murine dihydrofolate reductase enzyme by copper(I)-catalyzed azide-alkyne cycloaddition with retained activity. PLoS One 9: e9840
Bornscheuer, U. T. and M. Pohl (2001) Improved biocatalysts by directed evolution and rational protein design. Curr. Opin. Chem. Biol. 5: 137–143.
Steinrauf, L. K., J. A. Hamilton, B. C. Braden, J. R. Murrell, and M. D. Benson (1993) X-ray crystal structure of the Ala-109→Thr variant of human transthyretin which produces euthyroid hyperthyroxinemia. J. Biol. Chem. 268: 2425–2430.
Howard, E. I., R. Sanishvili, R. E. Cachau, A. Mitschler, B. Chevrier, P. Barth, V. Lamour, M. Van Zandt, E. Sibley, C. Bon, D. Moras, T. R. Schneider, A. Joachimiak, and A. Podjarny (2004) Ultrahigh resolution drug design I: details of interactions in human aldose reductase.inhibitor complex at 0.66 A. Proteins. 55: 792–804.
Yabe-Nishimura, C. (1998) Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacol. Rev. 50: 21–33.
Holliday, G. L., J. B. O. Mitchell, and J. M. Thornton (2009) Understanding the functional roles of amino acid residues in enzyme catalysis. J. Mol. Biol. 390: 560–577.
O’Hagan, D. (2008) Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 37: 308–319.
Purser, S., P. R. Moore, S. Swallow, and V. Gouverneur (2008) Fluorine in medicinal chemistry. Chem. Soc. Rev. 37: 320–330.
Bondi, A. (1964) van der Waals volumes and radii. J. Phys. Chem. 68: 441–451.
Budisa, N., W. Wenger, and B. Wiltschi (2010) Residue-specific global fluorination of Candida antarctica lipase B in Pichia pastoris. Mol. Biosyst. 6: 1630–1639.
Mathew, S., S. P. Nadarajan, T. Chung, H. H. Park, and H. Yun (2016) Biochemical characterization of thermostable ω-transaminase from Sphaerobacter thermophilus and its application for producing aromatic β- and γ-amino acids. Enzyme Microb. Technol. 87-88: 52–60.
Mathew, S., K. Deepankumar, G. Shin, E. Y. Hong, B. G. Kim, T. Chung, H. Yun (2016) Identification of novel thermostable ω-transaminase and its application for enzymatic synthesis of chiral amines at high temperature. RSC Adv. 6: 69257–69260.
Deepankumar, K., M. Shon, S. P. Nadarajan, G. Shin, S. Mathew, N. Ayyadurai, B. G. Kim, S. H. Choi, S. H. Lee, H. Yun (2014) Enhancing thermostability and organic solvent tolerance of ω-transaminase through global incorporation of fluorotyrosine. Adv. Synth. Catal. 356: 993–998.
Ohtake, K., A. Yamaguchi, T. Mukai, H. Kashimura, N. Hirano, M. Haruki, S. Kohashi, K. Yamagishi, K. Murayama, Y. Tomabechi, T. Itagaki, R. Akasaka, M. Kawazoe, C. Takemoto, M. Shirouzu, S. Yokoyama, and K. Sakamoto (2015) Protein stabilization utilizing a redefined codon. Sci. Rep. 5: 9762.
Hoesl, M. G., C. G. Acevedo-Rocha, S. Nehring, M. Royter, C. Wolschner, B. Wiltschi, and N. Budisa (2011) Lipase congeners designed by genetic code engineering. ChemCatChem. 3: 213–221.
Ugwumba, I. N., K. Ozawa, Z. Q. Xu, F. Ely, J. L. Foo, A. J. Herlt, C. Coppin, S. Brown, M. C. Taylor, D. L. Ollis, L. N. Mander, G. Schenk, N. E. Dixon, G. Otting, J. G. Oakeshott, and C. J. Jackson (2011) Improving a natural enzyme activity through incorporation of unnatural amino acids. J. Am. Chem. Soc. 133: 326–333.
Windle, C. L., K. J. Simmons, J. R. Ault, C. H. Trinh, A. Nelson, A. R. Pearson, and A. Berry (2017) Extending enzyme molecular recognition with an expanded amino acid alphabet. Proc. Natl. Acad. Sci. USA. 114: 2610–2615.
Kim, S., B. H. Sung, S. C. Kim, and H. S. Lee (2018) Genetic incorporation of l-dihydroxyphenylalanine (DOPA) biosynthesized by a tyrosine phenol-lyase. Chem. Commun. 54: 3002–3005.
Chen, Y., A. Loredo, A. Gordon, J. Tang, C. Yu, J. Ordonez, and H. Xiao (2018) A noncanonical amino acid-based relay system for site-specific protein labeling. Chem. Commun. 54: 7187–7190.
Ma, Y., H. Biava, R. Contestabile, N. Budisa, and M. L. Di Salvo (2014) Coupling bioorthogonal chemistries with artificial metabolism: intracellular biosynthesis of azidohomoalanine and its incorporation into recombinant proteins. Molecules. 19: 1004–1022.
Acknowledgments
This manuscript was supported by Konkuk University’s ‘Research Support for faculty on Sabbatical Leave’ program-2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Won, Y., Pagar, A.D., Patil, M.D. et al. Recent Advances in Enzyme Engineering through Incorporation of Unnatural Amino Acids. Biotechnol Bioproc E 24, 592–604 (2019). https://doi.org/10.1007/s12257-019-0163-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-019-0163-x