Abstract
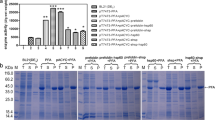
In vivo folding of many proteins can be facilitated by growth temperature, extent of induction, and molecular chaperones, which prevent over-expressed protein from being trapped into insoluble inclusion bodies. In the present report, we describe the role of molecular chaperones and growth temperature on the solubilization of overexpressed Cellobiose Phosphorylase (CBP) in Escherichia coli. The growth of host at low temperature enhanced enzyme in soluble fraction. Similarly, induction of target gene at low level of IPTG also yielded higher enzyme in soluble fraction. The synergistic effect of low temperature and induction on the prevention of inclusion bodies was also evident from our results. In addition, co-expression of the target gene with two types of molecular chaperones (GroESL and KODHsp) was also attempted. However, none of these chaperones enhanced the solubilization under in vivo conditions. Nevertheless, effective role of low growth temperature coupled with low level of induction appeared to be an attractive feature for producing recombinant protein.
Similar content being viewed by others
References
Tanaka, K., K. Kawaguchi, T. Imada, T. Ooi, and M. Arai (1995) Purification and properties of cellobiose phosphorylase from Clostridium thermocellum. J. Ferment. Bioeng. 79: 212–216.
Liu, A., H. Tomita, H. Li, H. Miyaki, C. Aoyagi, S. Kaneko, and K. Hayashi (1998) Cloning, sequencing, and expression of the cellobiose phosphorylase gene of Cellvibrio gilvus. J. Ferment. Bioeng. 85: 511–513.
Ohdan, K., K. Fujii, M. Yanase, V. Takaha, and T. Kuriki (2007) Phosphorylase coupling as a tool to convert cellobiose into amylose. J. Biotechnol. 127: 496–502.
Charoensakdi, R., S. Murakami, K. Aoki, V. Rimphanitchayakit, and T. Limpaseni (2006) Cloning and expression of cyclodextrin glycosyltransferase Gene from Paenibacillus sp. T16 isolated from hot spring soil in northern Thailand. J. Biochem. Mol. Bio. 40: 333–340.
Nidetzky, B., R. Griessler, A. Schwarz, and B. Splechtna (2004) Cellobiose phosphorylase from lulomonas uda gene cloning and expression in Escherichia coli, and application of the recombinant enzyme in a ‘glycosynthase-type’ reaction. J. Mol. Cata. B: Enzymatic 29: 241–248.
Ito, S., S. Hamada, K. Yamaguchi, S. Umene, H. Ito, H. Matsui, T. Ozawa, H. Taguchi, J. Watanabe, J. Wasaki, and S. Ito (2007) Cloning and sequencing of the cellobiose 2-epimerase gene from an obligatory anaerobe, Ruminococcus albus. Biochem. Biophys. Res. Commun. 360: 640–645.
Machida, S., Y. Yu, S. P. Singh, J. D. Kim, K. Hayashi, and Y. Kawata (1998) Overproduction of β-glucosidase in active form by an Escherichia coli system coexpressing the chaperonin GroEL/ES. FEMS Microbiol. Lett. 159: 41–46.
Singh, S. P., J. D. Kim, S. Machida, and K. Hayashi (2002) Overexpression and protein folding of a chimeric β-glucosidase constructed from obacterium tumefaciensand Cellvibrio gilvus. Ind. J. Biochem. Biophys. 39: 235–239.
Kim, D., S. Singh, S. Machida, Y. Chika, Y. Kawata, and K. Hayashi (1998) Importance of five amino acid residues at Cterminal region for the folding and stability of β-Glucosidase of Cellvibrio gilvus. J. Ferment. Bioeng. 85: 433–435.
Machida, S., S. Ogawa, S. Xiaohua, T. Takaha, K. Fujii, and K. Hayashi (2000) Cycloamylose as an efficient artificial chaperone for protein refolding. FEBS Lett. 486: 131–135.
Dodia, M. S., C. M. Rawal, H. G. Bhimani, R. H. Joshi, S. K. Khare, and S. P. Singh (2008) Purification and stability characteristics of an alkaline serine protease from a newly isolated haloalkaliphilic bacterium sp. AH-6. J. Ind. Microbiol. Biotechnol. 35: 121–131.
Dodia, M. S., H. G. Bhimani, C. M. Rawal, R. H. Joshi, and S. P. Singh (2008) Salt dependent resistance against chemical denaturation of alkaline protease from a newly isolated Haloalkaliphilic Bacillus sp. Bioresour. Technol. 99: 6223–6227.
Benech, R. O., X. Li, D. Patton, J. Powlowski, R. Storms, R. Bourbonnais, M. Paice, and A. Tsang (2007) Recombinant expression, characterization, and pulp prebleaching property of a Phanerochaete chrysosporium endo-β-1,4-mannanase. Enzy. Microb. Technol. 41: 740–747.
Maeda, Y., H. Koga, H. Yamada, T. Ueda, and T. Imoto (1995) Effective renaturation of reduced lysozyme by gentle removal of urea. Protein Eng. 8: 201–205.
Maeda, Y., H. Koga, H. Yamada, T. Ueda, and T. Imoto (1996) Effect of additives on the renaturation of reduced lysozyme in the presence of 4 M urea. Protein Eng. 9: 461–465.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S.P., Purohit, M.K., Aoyagi, C. et al. Effect of growth temperature, induction, and molecular chaperones on the solubilization of over-expressed cellobiose phosphorylase from Cellvibrio Gilvus under in vivo conditions. Biotechnol Bioproc E 15, 273–276 (2010). https://doi.org/10.1007/s12257-009-0023-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-009-0023-1