Summary
The biology of non-small cell lung cancer (NSCLC) is driven by a complex mutational landscape, and the detection of driver molecular alterations by next-generation sequencing is key for identification of druggable alterations. Thus, broad molecular profiling displays a standard-of-care approach particularly in patients with advanced adenocarcinoma at the time of the initial diagnosis, but also at the time of acquired resistance to tyrosine kinase inhibitors, guiding further treatment choices. Sequencing of plasma-circulating tumor DNA is of increasing importance in NSCLC diagnostics due to the easy accessibility, representing an optimal tool for longitudinal monitoring of the disease course.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide, with non-small cell lung cancer (NSCLC) being the most common type. In tumorigenesis, the transformation from normal airway epithelial cells to preinvasive lesions to highly malignant invasive cancer cells is determined by a variety of host and tumor intrinsic factors. The latter involves a dynamic genomic evolution that is characterized by accumulation of specific molecular alterations and an increased mutational burden over time [1]. There is an extensive intra-tumor heterogeneity within this mutational process and diverse mutations can act as substrates for clonal selection and expansion, constantly fueling tumor development as well as drug resistance [2]. The molecular understanding is continuously advancing and has led to the revolutionizing paradigm of “precision medicine” or “personalized cancer medicine,” aiming to find a selective targeted therapy for a specific tumoral molecular alteration. Thus, in-depth molecular profiling in NSCLC is state the art at the time point of the initial diagnosis, but also increasingly established at time points of progressive disease under established therapy to identify potential resistance genes or novel mutations. This article reviews the background, clinical importance, limitations, and future directions of molecular profiling in lung cancer.
The mutational process in NSCLC
NSCLC cells harbor a variety of genetic alterations at the mutational (e.g., point mutations, short insertions, deletions) and well as chromosomal (copy number alterations, e.g., amplifications, allelic imbalance, gene-fusions) level and the presence of these alterations is marked by a widespread intra-tumor heterogeneity. Alterations can be clonal (present in all cancer cells) or subclonal (present in a subset of cancer cells) and this discrimination of clonality has a clinical implication since targeting subclonal mutations may lead to reduced treatment efficacy. Nearly 75% of all NSCLCs undergo genome doubling (where the genome becomes tetraploid) in the founding clone, an important event that probably allows cancer cells to tolerate chromosomal instability later on [3]. Cancer driver events that occur early during cancer evolution are almost exclusively clonal. They are tumor-initiating, frequently associated with smoking carcinogens and are mostly specific for a certain histological subtype. For example, in adenocarcinoma, these early mutations or amplifications occur in BRAF, EGFR, or MET, while in squamous carcinoma there are NOTCH1 mutations or FGFR1 amplifications. TP53 mutations are mostly clonal and occur in both subtypes [2]. The clonal expression of these driver alterations explains the strong responses frequently observed across multiple tumor sites when therapeutically targeted. Late driver mutations are frequent (carried by over 75% of tumors), mostly subclonal and involved in tumor maintenance rather than initiation. They are not NSCLC subtype-specific and frequently affect genes that have also been observed in other tumor types (“pan-cancer genes”), for example, PIK3CA and NF1 [2]. Moreover, the acquisition of chromosomal instability (CIN; e.g., copy number change, loss or gain of alleles) is an ongoing process throughout tumor evolution and a hallmark of carcinogenesis. Tumor heterogeneity driven by chromosomal instability is associated with increased risk of tumor recurrence or death and may serve as a prognostic predictor [2].
Indications for molecular profiling
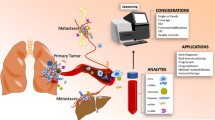
The vast majority of oncogene-addicted lung cancers are adenocarcinomas. Patients are generally younger and never- or long-time ex- (> 10 years) or light smokers (< 15 packyears). Female gender and east-Asian ethnicity show particular enrichment for EGRF-mutant and ALK translocated tumors. Molecular profiling is recommended after diagnosis and ideally before treatment initiation in all patients with advanced possible, probable, or definite adenocarcinoma as well as other non-squamous histologies (e.g., large cell; [4, 5]). Furthermore, molecular testing should be considered in young patients (< 50 years) independent of histology [6], never-smokers [7], and sarcomatoid histology (due to the high prevalence of MET exon-14-skipping mutations; [8]) Contrary to the latest ASCO and ESMO guidelines [4, 5], the 2021 NCCN guideline [9] also recommends to consider molecular testing in patients with squamous cell carcinoma independent of the mentioned typical clinical features of oncogene addiction. Testing for EGRF mutations in surgical resections of patients with early-stage (IB-IIIA) tumors may also be considered in order to evaluate for adjuvant osimertinib therapy [9]. Figure 1 displays important methods for tumor profiling.
Modern tumor profiling in lung cancer. (1) Tumor profiling from primary tumor tissue should include convectional histology including immunohistochemistry, fluorescence in situ hybridization (FISH), and molecular analysis by multi-gene panel-based next-generation sequencing from RNA or DNA. (2) Liquid biopsy by next-generation sequencing of circulating tumor DNA (ctDNA) can be used to screen for specific mutations (e.g., resistance mutations) or alternatively to apply multi-gene analysis. Figure created with biorender
Mutations of clinical relevance
In any solid cancer, molecular alterations can be used as predictive biomarkers (when having a corresponding specific targeted therapy that has been shown to improve outcome) or as prognostic biomarkers (indicating survival probability independent of therapy) [9]. Typically, key biomarkers do not overlap and most patients only have one actionable mutation [10]. In NSCLC, key molecular alterations include EGFR, BRAFV600E, KRASG12C, and ERBB2 (HER2) mutations, ALK, ROS1, NTRK 1/2/3, and RET fusions as well as MET exon-14 skipping mutations. Alterations of currently less clinical importance include BRCA 1/2, PIK3CA, and NRG1 [5]. To support the value of certain molecular alterations as actionable (druggable) targets and help clinicians to prioritize targets for precision medicine, the ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT) was developed. ESCAT is an evidence-based framework that ranks the match between a genomic alteration and a drug from level I (very good match, validated in clinical trials) to level X (lack of evidence) [11]. The ESMO guideline recommends screening ESCAT level I alterations (Table 1). A broad panel-based approach, typically by next-generation sequencing (NGS) from DNA or RNA (better in detection of fusion genes), is clearly preferred over multiple single-gene tests [5]. KRAS testing is not (yet) routinely recommended by ESMO (ESCAT IIB), but it should be noted that these recommendations were published before the approval of the KRASG12C tyrosine kinase inhibitor (TKI) sotorasib and will most likely be adapted in the next update. The discussion of NGS results in institutional molecular boards to select molecular-matched experimental therapies may improve the outcome of advanced cancer patients [12].
Limitations of molecular profiling
Tumors harbor an intensive heterogeneity on both the cellular as well as the molecular level. As such, many driver mutations are clonal in one tumor region but subclonal or even absent in another region, highlighting a major limitation of sampling single tumor regions, as is mostly done in the clinical setting (e.g., single tumor biopsies). For example, in the TRACERx study around 75% of subclonal mutations identified through multi-regional sequencing would have appeared clonal if only a single site had been biopsied. Applying multi-regional sequencing could help to define the clonality of mutations and to prioritize drug targets [2]. Currently, guidelines do not specifically discuss this limitation.
Molecular profiling to detect resistance genes
The occurrence of acquired resistance to TKIs is a frequent complication in NSCLC treatment, usually arising after 10–14 months of therapy (in the case of EGFR TKI; [13]). Several acquired resistance mutations have been described (Table 1). Current guidelines recommend to test for EGFR T790M exon 20 mutation in patients with EGFR-mutant adenocarcinoma after progression on EGFR TKIs when evaluating for third-generation TKI [4, 5]. Since osimertinib is now regularly used as first-line therapy, this recommendation loses importance and testing for other secondary genetic alteration as potential druggable targets could be performed. These include focal MET mutations and amplifications (15% of patients with first-line osimertinib resistance), HER2 amplifications (5% of patients with first-line osimertinib resistance), as well as BRAFV600E mutations [14]. Also, several secondary ALK resistance mutations have been described [15]. Currently non-druggable resistance genes include EGFR C797S (accounting for up to 26% of cases of osimertinib resistance), PIK3CA mutation/amplification, KRASG12D mutations, as well as long non-coding RNAs (lncRNA), e.g., MALAT1, and non-coding microRNAs (miRNAs; [13, 14]).
Molecular testing with cell-free DNA
Liquid biopsy enables the detection of tumor-derived gene alterations and mutations based on plasma-circulating tumor DNA (ctDNA), the tumor-specific form of cell-free-DNA (cfDNA), which is released by cancer cells through a combination or necrosis, apoptosis, and active secretion [16]. This flexible and minimally invasive method can be applied in various clinical contexts such as cancer screening and early diagnostics, in longitudinal monitoring of disease progression, relapse, and treatment response [17] as well as for molecular minimal residual disease (MRD) evaluation adding information to the conventional radiologic and clinical methods of cancer aftercare [17].
In NSCLC, the use of cfDNA has so far been recommended for EGFR testing in patients where tissue is insufficient for molecular testing or in the acquired TKI resistance situation to test for EGFR T790M mutations [4]. More recent guidelines by the International Association for the Study of Lung Cancer (IASLC; [18]) recommend cfDNA analysis by NGS to allow for a broad genomic interrogation instead of single hotspot mutations. IASLC recommend cfDNA at the time of diagnosis in advanced NSCLC (complementary to tissue) and at the time of acquired TKI resistance (before tissue re-testing). Further applications, such as serial monitoring of disease kinetics under therapy [18, 19] or evaluation of immunotherapy resistance genes such as PTEN and STK11 mutations [20], will likely be of importance in the near future.
Conclusion
The biology of NSCLC is driven by a complex mutational process and the detection of driver molecular alterations by modern NGS platforms is key for facilitating precision medicine. Importantly, if patients have both a high PD-L1 expression and a molecular biomarker, targeted therapy is typically recommended over immunotherapy, since targeted therapy shows higher response rates in the first-line setting, a better tolerability, and usually patients with oncogene-addicted tumors only show modest or slight responses to immunotherapy (although more research is needed; [21]). Therefore, molecular profiling displays a standard-of-care approach particularly in patients with adenocarcinoma, at the time of the first diagnosis and at the time of acquired TKI resistance. Liquid biopsy has entered clinical application in NSCLC diagnostics and might serve as parameter monitoring in the future.
References
Devarakonda S, Govindan R. Untangling the evolutionary roots of lung cancer. Nat Commun. 2019;10(1):2979. https://doi.org/10.1038/s41467-019-10879-6.
Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376(22):2109–21. https://doi.org/10.1056/NEJMoa1616288.
Dewhurst SM, McGranahan N, Burrell RA, et al. Tolerance of whole-genome doubling propagates chromosomal instability and accelerates cancer genome evolution. Cancer Discov. 2014;4(2):175–85. https://doi.org/10.1158/2159-8290.CD-13-0285.
Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J Clin Oncol. 2018;36(9):911–9. https://doi.org/10.1200/JCO.2017.76.7293.
Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(11):1491–505. https://doi.org/10.1016/j.annonc.2020.07.014.
Viñal D, Martínez D, Higuera O, de Castro J. Genomic profiling in non-small-cell lung cancer in young patients. A systematic review. ESMO Open. 2021;6(1):100045. https://doi.org/10.1016/j.esmoop.2020.100045.
Devarakonda S, Li Y, Martins Rodrigues F, et al. Genomic profiling of lung adenocarcinoma in never-smokers. J Clin Oncol. 2021;39(33):3747–58. https://doi.org/10.1200/JCO.21.01691.
Liu X, Jia Y, Stoopler MB, et al. Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol. 2016;34(8):794–802. https://doi.org/10.1200/JCO.2015.62.0674.
Ettinger DS, Wood DE, Aisner DL, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254–66. https://doi.org/10.6004/jnccn.2021.0013.
Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience. J Thorac Oncol. 2015;10(5):768–77. https://doi.org/10.1097/JTO.0000000000000516.
Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29(9):1895–902. https://doi.org/10.1093/annonc/mdy263.
Gambardella V, Lombardi P, Carbonell-Asins JA, et al. Molecular profiling of advanced solid tumours. The impact of experimental molecular-matched therapies on cancer patient outcomes in early-phase trials: the MAST study. Br J Cancer. 2021;125(9):1261–9. https://doi.org/10.1038/s41416-021-01502-x.
Zhang K, Yuan Q. Current mechanism of acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and updated therapy strategies in human nonsmall cell lung cancer. J Cancer Res Ther. 2016;12(Supplement):C131–C7. https://doi.org/10.4103/0973-1482.200613.
Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29:viii740. https://doi.org/10.1093/annonc/mdy424.063.
Pan Y, Deng C, Qiu Z, Cao C, Wu F. The resistance mechanisms and treatment strategies for ALK-rearranged non-small cell lung cancer. Front Oncol. 2021;11:713530. https://doi.org/10.3389/fonc.2021.713530.
Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223–38. https://doi.org/10.1038/nrc.2017.7.
Yan YY, Guo QR, Wang FH, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9:639233. https://doi.org/10.3389/fcell.2021.639233.
Rolfo C, Mack P, Scagliotti GV, et al. Liquid biopsy for advanced NSCLC: a consensus statement from the International Association for the Study of Lung Cancer. J Thorac Oncol. 2021;16(10):1647–62. https://doi.org/10.1016/j.jtho.2021.06.017.
Kwon M, Ku BM, Olsen S, et al. Longitudinal monitoring by next-generation sequencing of plasma cell-free DNA in ALK rearranged NSCLC patients treated with ALK tyrosine kinase inhibitors. Cancer Med. 2022; https://doi.org/10.1002/cam4.4663.
Guibert N, Jones G, Beeler JF, et al. Targeted sequencing of plasma cell-free DNA to predict response to PD1 inhibitors in advanced non-small cell lung cancer. Cancer Treat Res. 2019;137:1–6. https://doi.org/10.1016/j.lungcan.2019.09.005.
Addeo A, Passaro A, Malapelle U, Luigi Banna G, Subbiah V, Friedlaender A. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat Rev. 2021;96:102179. https://doi.org/10.1016/j.ctrv.2021.102179.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Author information
Authors and Affiliations
Contributions
LH and LN drafted the manuscript, LN prepared the figure, LH prepared the table, AP, DW and GP critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
G. Pall has received speaker fees and is an advisory board member of Astra Zeneca, Roche, Böhringer Ingelheim, Pfizer, Takeda, Lilly, Janssen, Novartis, Merck, Amgen, Daichii-Sankyo. D. Wolf has received speaker fees from MSD, Pfizer, Roche, Novartis, BMS/Celgene and Astra Zeneca. A. Pircher has received speaker fees and honoraria for advisory boards from Astra Zeneca, BMS, Roche, Pfizer, Takeda and MSD. L. Nagl and L. Horvath have received travel fees from MSD.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagl, L., Pall, G., Wolf, D. et al. Molecular profiling in lung cancer. memo 15, 201–205 (2022). https://doi.org/10.1007/s12254-022-00824-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-022-00824-7