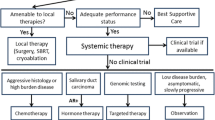
Summary
Salivary gland cancers (SGC) are a rare and heterogeneous group of malignancies. Most frequently tumors arise in the parotid gland. The most common histologic subtypes are adenoid cystic carcinoma (ACC) and mucoepidermoid carcinoma (MEC). Rare subtypes include salivary ductal carcinoma (SDC), mammary analogue secretory carcinoma (MASC) and adenocarcinoma not other specified (AC NOS). For locally advanced or metastatic disease, chemotherapy has been the mainstay of therapy. The course of disease differs markedly between the subtypes, especially ACC usually presents as slowly progressing disease. Due to the rarity of these tumors only small phase I/II studies exist, which report efficacy of cytotoxic regimens in advanced SGC. However, due to advances in the understanding of tumor biology and molecular testing, drugable genetic changes like androgen receptor (AR) status, HER2/neu overexpression and neurotrophic tyrosine receptor kinase (NTRK) gene fusion have evolved as potential therapy targets in subsets of SGC. Consequently therapy with androgen receptor blockade (ARB) can be offered to patients with AR expressing tumors. Anti-HER2 therapy with trastzumab is an option for the treatment of tumors with overexpression of HER2/neu and finally NTRAK inhibitors can be used for tumors harboring a NTRK gene fusion. Taken together, due to the small number of patients, data from large phase III studies for the treatment of SGC are missing. However, promising targeted therapy approaches have been recently undertaken.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Epidemiology
SGC represent approximately 6–8% of all head and neck tumors [1]. The incidence in Europe is around 4 per 100,000 people [2]. The majority of tumors arises in the parotid gland (approximately 80%). However, only one quarter of these tumors are malignant. The rate of neoplasms is substantially higher in tumors of the submandibular, sublingual and minor salivary glands [3].
Benign and malignant salivary gland tumors are classified according the 2017 WHO system. Mucoepidermoid carcinoma (MEC) and adenoid cystic carcinoma (ACC) represent the most frequent salivary gland carcinoma (SGC). SGC are classified according their clinical behavior into low, intermediate and high grade, with the latter displaying a more aggressive phenotype [1].
The aim of this review is to recapitulate treatment options for ACC and non-ACC, especially focusing on targeted therapies which are already available for subsets of SGCs.
ACC
In the majority of cases, ACC is characterized by its slowly progressing nature. Patients with lung metastases only seem to have better outcomes than patients with more widespread disease [4, 5]. The median over survival (mOS) of patients with metastatic ACC is reported to be approximately 3 years [5,6,7,8,9].
Especially patients presenting with oligometastatic disease can obtain great benefit from definitive locoregional therapies. In a series of 83 oligometastatic head and neck cancer patients, 16 patients with metastatic ACC were included. The 5‑year OS after definitive locoregional therapy was 84% in the ACC subset [8].
In patients with slowly progressing disease or without symptom burden due to their malignant disease, it may be feasible to delay treatment due to the indolent course of disease.
Active agents that were tested mainly in phase II studies include cisplatin [10, 11], mitoxantron [12, 13], epirubicin [14] and vinorelbine [15]. However, it has to be kept in mind that disease stabilization was far more common in all this studies than an objective response, which might be due to the slowly progressing nature of this disease [9].
Combination therapy with cisplatin, doxorubicin and cyclophosphamide (CAP) provided overall response rates (ORR) between 18–33% in a total of 4 studies [16,17,18,19]. It has to be kept in mind that the triplet therapy is toxic. Replacing cyclophosphamide with 5‑fluorouracil (PAF) led to ORR of 33% in ACC [20]. Combining 4 cytotoxic drugs (cisplatin, doxorubicin, 5‑fluorouracil and cyclophosphamide; CAPF) led to an increased ORR (43%, 3 out of 7 patients) at the cost of severe toxicity. As a consequence this regimen should not be recommended [21]. A doublet therapy consisting of cisplatin and an anthracycline leads to ORR of roughly 25% [9]. A substitution of cisplatin with the less oto- and nephrotoxic carboplatin may be even less effective, as it was reported in two studies that all ACC patients that received carboplatin instead of cisplatin had no objective response to therapy [22, 23]. Further agents that have only limited activity include gemcitabine and paclitaxel [9, 24]. Taken together, results of chemotherapy in ACC are disappointing with only low response rates and no advantage in quality of life. Furthermore, no responses have yet been reported in previously treated ACC patients [2].
Targeted therapy in ACC
Given the fact that chemotherapy has only limited efficacy in ACC, approaches targeting molecular targets were investigated. Between 60–90% of ACC overexpress c‑kit [25, 26]. However, all studies targeting c‑kit with either imatinib or dasatinib showed disappointing results with ORR below 5% [27,28,29,30]. In addition, neither therapies targeting the epidermal growth factor receptor (EGFR) with gefitinib [31], cetuximab [32] or lapatinib [33], nor the vascular endothelial growth factor (VEGF) 1&2 with sunitinib [34] showed meaningful response rates in previously treated patients. However, response rates of at least 9–11% were reported for sorafenib and axitinib in small phase II studies [31, 35]. Nevertheless, progression after first-line therapy was not required as an inclusion criterion.
Recently, lenvatinib showed modest activity in a phase II trial of 33 previously treated patients with ACC. The partial response rate was 16%, and in 75% of patients stable disease was achieved. Consequently, lenvatinib represents a possible option for second-line therapy after chemotherapy in ACC.
A more aggressive subset of ACC is defined by NOTCH homologue 1 (NOTCH1) mutation, which occurs in 26% of metastatic ACC [36]. A phase II study investigating the efficacy of AL101, a NOTCH inhibitor, is still ongoing (NCT03691207).
MEC, salivary duct carcinoma (SDC) and adenocarcinoma not other specified (AC NOS)
In the absence of driver mutations, chemotherapy can be offered to these patients. Again, the available data only derive from small phase II studies including various histological subtypes. Single agents that showed efficacy are cisplatin, paclitaxel [37] and vinorelbine [15]. Presumably more active are combination therapies. These include CAP [16,17,18, 38, 39] PAF [20, 40], cisplatin/gemcitabine [22] and carboplatin/paclitaxel [41].
Beside the limited data on chemotherapy in this subset of SGC, targeted therapies are under investigation. Overexpression or amplification of human epidermal growth factor receptor 2 (HER2) is reported in up to 30% of MEC, in 40% of SDC and in 20% of AC NOS [42,43,44,45]. Overexpression is defined as IHC 3+, or IHC 2+ plus FISH HER2/chromosome 17 ratio >2. Trastuzumab as monotherapy has only limited efficacy with ORR of around 10% [46,47,48]. A very recent phase II study included 57 SDC patients with HER2 overexpression. The combination treatment of trastuzumab and docetaxel showed promising results with an ORR of 70.2% and mOS 39.7 months. The most common toxicities were hematological adverse events with anemia and neutropenia. In addition treatment with ado-trastuzumab-emtansine (TDM-1) might pose a subsequent option after trastuzumab therapy. A phase II study showed some activity in SDC without previous anti-HER2 therapy [49]. Furthermore, preliminary data of a phase II basket trial presented at ASCO 2019 reported ORR upon TDM‑1 of up to 90% in SDC previously treated with trastuzumab (NCT02675829).
AR expression is a frequent event in SDC and AC NOS, with rates of up to 90% [50,51,52]. Androgen deprivation therapy (ADT) is an option for those patients with AR expression SGC. A small phase II study enrolled 36 patients for combined therapy with bicalutamide and leuprorelin: 94% of patients had SDC and 6% AC NOS. The ORR was 41.7% and the mOS 30.5 months. Toxicities were limited and well manageable [51]. In addition a randomized phase II study comparing chemotherapy (cisplatin/doxorubicin or carboplatin/paclitaxel) vs. ADT (bicalutamide/leuprorelin) in patients with SDC or AC NOS in the first-line setting is ongoing (NCT01969578).
MASC
Mammary analogue secretory carcinoma (MASC) represents a rare subset of SGC and shows similarities to secretory carcinomas of the breast, especially regarding activating gene fusions of NTRK3 gene, with the ETV6-NTRK3 being the most common occurring in up to 99% of all MASCs [53, 54]. According to the preliminary results of phase I/II studies, both the FDA and EMEA approved larotrectinib for solid malignancies with NTRK fusion.
In a study with 55 patients an NTRK gene fusion positive solid cancers 12 patients with MASC were included. ORR was 83% (n = 10) and 25% (n = 2) experienced a complete remission [55]. In addition, entrectinib, another NTRK inhibitor proved its efficacy in a pooled analysis of three single arm trials including 54 patients. Seven MASC patients were included, and the ORR in this subset was 86% [56].
Both compounds are well tolerated and no discontinuation due to adverse events occurred [55, 56]. Due to the clinical efficacy and its good safety profile, entrectinib received accelerated approval by the FDA.
Checkpoint inhibitor therapy
The only available data come from a small phase Ib study (KEYNOTE 028) which included 26 programmed death ligand‑1 (PD-L1) positive SGC patients. The overall response rate (ORR) was modest with 12%. In total, 3 patients achieved a partial response, and no complete responses were reported. The median PFS was 4 months and the mOS 13 months [57]. In addition, 3 small studies were presented at the ASCO 2019 utilizing CIT in SGCs. However, the results were disappointing. A combination of nivolumab and ipilimumab in recurrent or metastatic ACC showed an ORR of 6% [58]. Another study in ACC patients compared pembrolizumab with or without hypofractionated radiotherapy. Of the 20 included patients, none reached an objective tumor response. However, 65% of patients reached a stable disease (SD) [58]. Another multicentered phase II study included 46 ACC and 52 SGC patients for nivolumab therapy. The reported mPFS was 4.8 months for ACC patients and 1.8 months for SGC patients [58]. Further studies including SGC patients for checkpoint inhibitor therapy (CIT) have completed recruitment, but results are still lacking (KEYNOTE 158 NCT02628067) and NISCAHN (NCT03132038).
Conclusion
SGC are a heterogeneous and rare group of malignancies. Chemotherapy showed minor efficacy and the evaluation of therapy success is limited by the small number of patients. Targeted therapies are on the rise and potential molecular targets such as HER2, NTRK and AR do exist. However, SGC patients should be included into clinical trials to further explore potential therapeutic options.
References
Guzzo M, Locati LD, Prott FJ, Gatta G, McGurk M, Licitra L. Major and minor salivary gland tumors. Crit Rev Oncol Hematol. 2010;74(2):134–48.
Alfieri S, Granata R, Bergamini C, Resteghini C, Bossi P, Licitra LF, et al. Systemic therapy in metastatic salivary gland carcinomas: A pathology-driven paradigm? Oral Oncol. 2017;66:58–63.
Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8(3):177–84.
Gao M, Hao Y, Huang MX, Ma DQ, Luo HY, Gao Y, et al. Clinicopathological study of distant metastases of salivary adenoid cystic carcinoma. Int J Oral Maxillofac Surg. 2013;42(8):923–8.
Sung MW, Kim KH, Kim JW, Min YG, Seong WJ, Roh JL, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2003;129(11):1193–7.
Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174(5):495–8.
van der Wal JE, Becking AG, Snow GB, van der Waal I. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck. 2002;24(8):779–83.
Liu D, Labow DM, Dang N, Martini N, Bains M, Burt M, et al. Pulmonary metastasectomy for head and neck cancers. Ann Surg Oncol. 1999;6(6):572–8.
Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. 2011;12(8):815–24.
Licitra L, Marchini S, Spinazze S, Rossi A, Rocca A, Grandi C, et al. Cisplatin in advanced salivary gland carcinoma. A phase II study of 25 patients. Cancer. 1991;68(9):1874–7.
Schramm VL Jr., Srodes C, Myers EN. Cisplatin therapy for adenoid cystic carcinoma. Arch Otolaryngol. 1981;107(12):739–41.
Verweij J, de Mulder PH, de Graeff A, Vermorken JB, Wildiers J, Kerger J, et al. Phase II study on mitoxantrone in adenoid cystic carcinomas of the head and neck. Ann Oncol. 1996;7(8):867–9.
Mattox DE, Von Hoff DD, Balcerzak SP. Southwest Oncology Group study of mitoxantrone for treatment of patients with advanced adenoid cystic carcinoma of the head and neck. Invest New Drugs. 1990;8(1):105–7.
Vermorken JB, Verweij J, de Mulder PH, Cognetti F, Clavel M, Rodenhuis S, et al. Epirubicin in patients with advanced or recurrent adenoid cystic carcinoma of the head and neck: a phase II study of the EORTC Head and Neck Cancer Cooperative Group. Ann Oncol. 1993;4(9):785–8.
Airoldi M, Pedani F, Succo G, Gabriele AM, Ragona R, Marchionatti S, et al. Phase II randomized trial comparing vinorelbine versus vinorelbine plus cisplatin in patients with recurrent salivary gland malignancies. Cancer. 2001;91(3):541–7.
Creagan ET, Woods JE, Rubin J, Schaid DJ. Cisplatin-based chemotherapy for neoplasms arising from salivary glands and contiguous structures in the head and neck. Cancer. 1988;62(11):2313–9.
Dreyfuss AI, Clark JR, Fallon BG, Posner MR, Norris CM Jr., Miller D. Cyclophosphamide, doxorubicin, and cisplatin combination chemotherapy for advanced carcinomas of salivary gland origin. Cancer. 1987;60(12):2869–72.
Belani CP, Eisenberger MA, Gray WC. Preliminary experience with chemotherapy in advanced salivary gland neoplasms. Med Pediatr Oncol. 1988;16(3):197–202.
Licitra L, Cavina R, Grandi C, Palma SD, Guzzo M, Demicheli R, et al. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma. A phase II trial of 22 patients. Ann Oncol. 1996;7(6):640–2.
Venook AP, Tseng A Jr., Meyers FJ, Silverberg I, Boles R, Fu KK, et al. Cisplatin, doxorubicin, and 5‑fluorouracil chemotherapy for salivary gland malignancies: a pilot study of the Northern California Oncology Group. J Clin Oncol. 1987;5(6):951–5.
Dimery IW, Legha SS, Shirinian M, Hong WK. Fluorouracil, doxorubicin, cyclophosphamide, and cisplatin combination chemotherapy in advanced or recurrent salivary gland carcinoma. J Clin Oncol. 1990;8(6):1056–62.
Laurie SA, Siu LL, Winquist E, Maksymiuk A, Harnett EL, Walsh W, et al. A phase 2 study of platinum and gemcitabine in patients with advanced salivary gland cancer: a trial of the NCIC Clinical Trials Group. Cancer. 2010;116(2):362–8.
Ross PJ, Teoh EM, A’Hern RP, Rhys-Evans PH, Harrington KJ, Nutting CM, et al. Epirubicin, cisplatin and protracted venous infusion 5‑Fluorouracil chemotherapy for advanced salivary adenoid cystic carcinoma. Clin Oncol (R Coll Radiol). 2009;21(4):311–4.
van Herpen CM, Locati LD, Buter J, Thomas J, Bogaerts J, Lacombe D, et al. Phase II study on gemcitabine in recurrent and/or metastatic adenoid cystic carcinoma of the head and neck (EORTC 24982). Eur J Cancer. 2008;44(17):2542–5.
Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N, et al. The mutational landscape of adenoid cystic carcinoma. Nat Genet. 2013;45(7):791–8.
Holst VA, Marshall CE, Moskaluk CA, Frierson HF Jr.. KIT protein expression and analysis of c‑kit gene mutation in adenoid cystic carcinoma. Mod Pathol. 1999;12(10):956–60.
Hotte SJ, Winquist EW, Lamont E, MacKenzie M, Vokes E, Chen EX, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c‑kit: a Princess Margaret Hospital phase II consortium study. J Clin Oncol. 2005;23(3):585–90.
Ochel HJ, Gademann G, Rocken C, Wordehoff H. Effects of imatinib mesylate on adenoid cystic carcinomas. Anticancer Res. 2005;25(5):3659–64.
Pfeffer MR, Talmi Y, Catane R, Symon Z, Yosepovitch A, Levitt M. A phase II study of Imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol. 2007;43(1):33–6.
Wong SJ, Karrison T, Hayes DN, Kies MS, Cullen KJ, Tanvetyanon T, et al. Phase II trial of dasatinib for recurrent or metastatic c‑KIT expressing adenoid cystic carcinoma and for nonadenoid cystic malignant salivary tumors. Ann Oncol. 2016;27(2):318–23.
Locati LD, Bossi P, Perrone F, Potepan P, Crippa F, Mariani L, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: a phase II study. Oral Oncol. 2009;45(7):574–8.
Jakob JA, Kies MS, Glisson BS, Kupferman ME, Liu DD, Lee JJ, et al. Phase II study of gefitinib in patients with advanced salivary gland cancers. Head Neck. 2015;37(5):644–9.
Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007;25(25):3978–84.
Chau NG, Hotte SJ, Chen EX, Chin SF, Turner S, Wang L, et al. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol. 2012;23(6):1562–70.
Thomson DJ, Silva P, Denton K, Bonington S, Mak SK, Swindell R, et al. Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck. Head Neck. 2015;37(2):182–7.
Ho AS, Ochoa A, Jayakumaran G, Zehir A, Valero Mayor C, Tepe J, et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J Clin Invest. 2019;129(10):4276–89.
Gilbert J, Li Y, Pinto HA, Jennings T, Kies MS, Silverman P, et al. Phase II trial of taxol in salivary gland malignancies (E1394): a trial of the Eastern Cooperative Oncology Group. Head Neck. 2006;28(3):197–204.
Alberts DS, Manning MR, Coulthard SW, Koopmann CF Jr., Herman TS. Adriamycin/cis-platinum/cyclophosphamide combination chemotherapy for advanced carcinoma of the parotid gland. Cancer. 1981;47(4):645–8.
Eisenberger MA. Supporting evidence for an active treatment program for advanced salivary gland carcinomas. Cancer Treat Rep. 1985;69(3):319–21.
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30.
Airoldi M, Fornari G, Pedani F, Marchionatti S, Gabriele P, Succo G, et al. Paclitaxel and carboplatin for recurrent salivary gland malignancies. Anticancer Res. 2000;20(5C):3781–3.
Alotaibi AM, Alqarni MA, Alnobi A, Tarakji B. Human epidermal growth factor receptor 2 (HER2/neu) in salivary gland carcinomas: a review of literature. J Clin Diagn Res. 2015;9(2):ZE4–8.
Glisson B, Colevas AD, Haddad R, Krane J, El-Naggar A, Kies M, et al. HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res. 2004;10(3):944–6.
Jaehne M, Roeser K, Jaekel T, Schepers JD, Albert N, Loning T. Clinical and immunohistologic typing of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103(12):2526–33.
Weed DT, Gomez-Fernandez C, Pacheco J, Ruiz J, Hamilton-Nelson K, Arnold DJ, et al. MUC4 and ERBB2 expression in major and minor salivary gland mucoepidermoid carcinoma. Head Neck. 2004;26(4):353–64.
Haddad R, Colevas AD, Krane JF, Cooper D, Glisson B, Amrein PC, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003;39(7):724–7.
Limaye SA, Posner MR, Krane JF, Fonfria M, Lorch JH, Dillon DA, et al. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist. 2013;18(3):294–300.
Nabili V, Tan JW, Bhuta S, Sercarz JA, Head CS. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck. 2007;29(10):907–12.
Jhaveri KL, Wang XV, Makker V, Luoh SW, Mitchell EP, Zwiebel JA, et al. Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann Oncol. 2019;30(11):1821–30.
Fan CY, Melhem MF, Hosal AS, Grandis JR, Barnes EL. Expression of androgen receptor, epidermal growth factor receptor, and transforming growth factor alpha in salivary duct carcinoma. Arch Otolaryngol Head Neck Surg. 2001;127(9):1075–9.
Fushimi C, Tada Y, Takahashi H, Nagao T, Ojiri H, Masubuchi T, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979–84.
Szewczyk M, Marszalek A, Sygut J, Golusinski P, Golusinski W. Prognostic markers in salivary gland cancer and their impact on survival. Head Neck. 2019;41(9):3338–47.
Skalova A, Vanecek T, Majewska H, Laco J, Grossmann P, Simpson RH, et al. Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, beta-catenin, EGFR, and CCND1 genes. Am J Surg Pathol. 2014;38(1):23–33.
Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378(8):731–9.
Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–82.
Cohen RB, Delord JP, Doi T, Piha-Paul SA, Liu SV, Gilbert J, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 study. Am J Clin Oncol. 2018;41(11):1083–8.
Doescher J, Schuler PJ, Greve J, Meyer MF, Weissinger S, Hoffmann TK, et al. Salivary gland malignancies-highlights of the 2019 ASCO Annual Meeting. HNO. 2019;67(12):931–4.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Minichsdorfer received travel grants from: Roche, BMS, MSD, Merck and honoraria from BI, Merck.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Minichsdorfer, C. Systemic therapy for metastatic salivary gland tumors—challenges and novel concepts. memo 13, 400–404 (2020). https://doi.org/10.1007/s12254-020-00614-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-020-00614-z