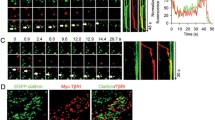
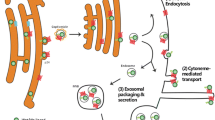
Abstract
Transforming growth factor β (TGF-β) superfamily consists of numerous cytokins that regulate various cellular processes. TGF-β, the prototype of the family, signals through its cell surface serine/threonin kinase receptors and besides its role in cell differentiation, migration, adhesion etc. it is also able to induce epithelial-mesenchymal (EMT) transition via both Smad- pathway and MAPK- pathway. Among the different types of epithelial-mesenchymal transition, type II that is described to be associated with wound healing, tissue regeneration, organ fibrosis and is induced upon inflammatory stimuli. It can be triggered by secretion of growth factors such as TGF-β, EGF. Different endocytic routes are used for the internalization of TGF-β ligand and its receptors and these pathways can control the activity of downstream events. Internalization via clathrin-coated vesicles promotes the signaling while the caveola-mediated endocytosis plays important role in the termination of the events, although the steps of the latter event are less clear. The early endosome is considered a clue compartment in promoting the signaling. Recently published data suggest that the early endosome plays crucial role in the termination of the TGFβ signaling as well. It is not only maintain a special environment for the effective signaling but can direct the internalized cargos towards degradative pathways (multivesicular bodies, lysosomes).




Similar content being viewed by others
References
Massagué J (1998) TGF-β signal transduction. Annu Rev Biochem 67:753–91
Shook D, Keller R (2003) Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev 120:1351–83
Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Müller GA, Neilson EG (2002) Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int 61:1714–28
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–28
Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J (1994) Mechanism of activation of TGF-β receptor. Nature 370:341–47
Shi Y, Massagué J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113:685–700
Moustakas A, Souchelnytskyi S, Heldin CH (2001) Smad regulation in TGF-β signal transduction. J Cell Sci 11:4359–69
Zhang Y, Chang C, Gehling JD, Hemmati-Brivanlou A, Derynck R (2001) Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc Natl Acad Sci U S A 98:974–79
Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana L (1998) SARA, a FYVE domain protein that recruits Smad2 to the TGF-β receptor. Cell 95:779–91
Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana L (2003) Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol 5:410–21
Chen YG (2009) Endocytic regulation of TGF-β signaling. Cell Res 19:58–70
Chen YG, Wang Z, Ma Y, Zhang L, Lu Z (2007) Endofin, a FYVE domain protein, interacts with Smad4 and facilitates transforming growth factor-beta signaling. J Biol Chem 282:9688–96
Gillooly DJ, Simonsen A, Stenmark H (2001) Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem J 355:249–58
Itoh F, Divecha N, Brocks L, Brocks L, Oomen L, Janssen H, Calafar J, Itoh S, Dijke PP (2002) The FYVE domain in Smad anchor for receptor activation (SARA) is sufficient for localization of SARA in early endosomes and regulates TGF-beta/Smad signalling. Genes Cells 7:321–31
Le Roy C, Wrana L (2005) Clathrin- and non-clathrin- mediated endocytic regulation of cell signalling. Nat Rev Cell Biol 6:112–26
Bonifacino JS, Lippincott-Schwartz J (2003) Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol 4:409–14
Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72:395–447
Hayes S, Chawla A, Corvera S (2002) TGFβ receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J Cell Biol 158:1239–49
Penheiter SG, Mitchell H, Garamszegi N, Edens M, Dore JJJ, Leof EB (2002) Internalization-dependent and –independent requirements for trasfotming growth factor beta receptor signaling via the Smad pathway. Mol Cell Biol 22:4750–59
Xu L, Chen YG, Massagué J (2000) The nuclear import function of Smad2 is masked by SARA and unmasked by TGFb-dependent phosphorylation. Nat Cell Biol 2:559–62
Heldin CH, Moustakas A (2012) Role of Smads in TGFβ signaling. Cell Tissue Res 347:21–36
Kurisaki A, Kose S, Yoneda Y, Heldin CH, Moustakas A (2001) Transforming growth factor-beta induces nuclear import of Smad3 in an importin-beta1 and Ran-dependent manner. Mol Biol Cell 12:1079–91
Xiao Z, Latek R, Lodish HF (2003) An extended bipartite nuclear localization signal in Smad4 is required for its nuclear import and transcriptional activity. Oncogene 22:1057–69
Mitchell H, Choudhury A, Pagano RE, Leof EB (2004) Ligand-dependent and –independent transforming growth factor-beta receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol Biol Cell 15:4166–78
Lisanti MP, Scherer P, Tang ZL, Sargiacomo M (1994) Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol 4:231–35
Couet J, Li S, Okamoto T, Scherer P, Lisanti MP (1997) Molecular and cellular biology of caveolae: paradoxes and plasticities. Trends Cardiovasc Med 7:103–10
Pelkmans L, Kartenbeck J, Helenius A (2001) Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular transport pathway to the ER. Nat Cell Biol 3:475–83
Nicols BJ (2002) A distinct class of endosome mediates clathrin- independent endocytosis to the Golgi complex. Nat Cell Biol 4:374–78
Parton RG, Simons K (2007) The multiple faces of caveolae. Nat Rev Mol Cell Biol 8:185–94
Kiss AL, Botos E (2009) Endocytosis via caveolae: alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J Cell Mol Med 13:1228–37
Hayer A, Stoaber M, Ritz D, Engel S, Meyer HH, Helenius A (2010) Caveolin-1 is ubiquitinated and targeted to intraluminal vesicles in endolysosomes for degradation. J Cell Biol 191:615–29
Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, PtP D (1997) Identification of Smad7, a TGF-beta inducible antagonist of TGF-beta signalling. Nature 389:631–35
Hayashi H, Abdollah S, Qui Y, Cai J, Xu JJ, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA Jr, Wrana JL, Falb D (1997) The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell 89:1165–73
Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG (2007) Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol 27:4488–99
Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL (2000) Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF-beta receptor for degradation. Mol Cell 6:1365–75
Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K (2001) Smurf1 interacts with transforming growth factor-beta type-I receptor through Smad7 and induces receptor degradation. J Biol Chem 276:12477–80
Itoh S, Landström M, Hermansson A, Itoh F, Heldin CH, Heldin NE, PtP D (1998) Transforming growth factor beta1 induces nuclear export of inhibitory Smad7. J Biol Chem 273:29195–201
Kowanetz M, Lönn P, Vanlandewijck M, Kowanetz K, Heldin CH, Moustakas A (2008) TGFbeta induces SIK to negatively regulate type I receptor kinase signaling. J Cell Biol 182:655–62
Bizet AA, Liu K, Tran-Khanh N, Saksena A, Vorstenbosch J, Finnson KW, Buschmann MD, Philip A (2011) The TFG-β co-receptor, CD109, promotes internalization and degradation of TGF-β receptors. Biochim Biophys Acta 1813:742–53
Mukhopadhyay D, Riezman H (2007) Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315:201–205
Ciechanover A (2005) Proteolysis: from the lysosome to ubiquitin and the proteasome. Mol Cel Biol 6:79–86
Felder S, Miller K, Moshren G, Ullrich A, Schlessinger J, Hopkins CR (1990) Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell 61:623–34
Gruenberg J, Maxfield F (1995) Membrane transport in the endocytic pathway. Curr Op Cell Biol 7:552–63
von Gersdoff G, Susztak K, Rezvani F, Bitzer M, Liang D, Böttinger EP (2000) Smad3 and Smad4 mediate transcriptional activation of the human Smad7 promoter by transforming growth factor beta. J Biol Chem 275:11320–26
Katzmann DJ, Babst M, Emr DS (2001) Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106:145–55
Soond SM, Chantry A (2011) How ubiqitination regulates the TGF-β signalling pathway: new insights and new players. Bioessays 33:749–58
Ito I, Hanyu A, Wayama M, Goto N, Katsuno Y, Kawasaki S, Nakajima Y, Kajiro M, Komatsu Y, Fujimura A, Hirota R, Murayama A, Kimura K, Imamura T, Yanagisawa J (2010) Estrogen inhibits transforming growth factor β signaling by promoting Smad2/3 degradation. J Biol Chem 285:14747–55
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 424:577–584
Moustakas A, Heldin CH (2005) Non-Smad TGF-β signals. J Cell Sci 118:3573–3584
Chuderland D, Seger R (2005) Protein-protein interactions in the regulation of the extracellular signal-regulated kinase. Mol Biothechnol 29:57–74
Adachi M, Fukuda M, Nishida E (2000) Nuclear export of MAP kinase (Erk) involves a MAP kinase kinase (MEK)-dependent active transport mechanism. J Cell Biol 148:849–856
Chen RH, Sarnecki C, Blenis J (1992) Nuclear localization and regulation of erk- and rsk-encoded protein kinase. Mol Cell Biol 12:915–927
Kamaraju AK, Roberts AB (2005) Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-β-mediated Smad-dependent growth inhibition of human breast carcinom cells in vivo. J Biol Chem 280:1024–1036
Mori S, Matsuzaki K, Yoshida K, Furukawa F, Tahashi Y, Yamagata H, Sekimoto G, Seki T, Matsui H, Nishizawa M, Fujisawa J, Okazaki K (2004) TGF-β and HGF transmit the signals through JNK-dependent Smad2/3 phosphorylation at the linker region. Oncogene 23:7416–7429
Kretzschmar M, Doody J, Timokhina I, Massagué J (1999) A mechanism of repression of TGF-β/Smad signaling by oncogenic Ras. Genes Dev 13:804–816
Engel M, McDonnell MA, Law BK, Moses HL (1999) Interdependent Smad and JNK signaling in trasforming growth factor-β-mediated transcription. J Biol Chem 274:37413–37420
Kang JS, Liu C, Derynck R (2009) New regulatory mechanisms of TGF-β receptor function. Trends Cell Biol 19:385–394
Galliher AJ, Schiemann WP (2007) Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during brest cancer cell proliferation and invasion. Cancer Res 67:3752–3758
Taub N, Teis D, Ebner HL, Hess MW, Huber LA (2007) Late endosomal traffic of epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol Biol Cell 18:4698–4710
Zehorai E, Yao Z, Plotnikov A, Seger R (2010) The subcellular localization of MEK and ERK-A novel nuclear translocation signal (NTS) paves a way to nucleus. Mol Cell End 314:213–220
Zuo W, Chen YG (2009) Specific activation of mitogen-activated protein kinase by transforming growth factor-β receptors in lipid rafts is required for epithelial cell plasticity. Mol Biol Cell 20:1020–1029
Galmiche A, Fueller J, Santel A, Krohne G, Witting I, Doye A, Rolando M, Flatau G, Lemichez E, Rapp UR (2008) Isoform-specific interaction of C_RAF with mitochondria. J Biol Chem 283:14857–14866
Adachi M, Fukuda M, Nishida E (1999) Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J 18:5347–5358
Fukuda M, Gotoh I, Gotoh Y, Nishida E (1996) Cytoplasmic localization of MAP kinase kinase directed by its N-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem 271:20024–20028
Fukuda M, Gotoh Y, Nishida E (1997) Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J 16:1901–1908
Acknowledgments
The quality of the manuscript was greatly enhanced by the gracious help of Professor Pál Röhlich. The authors are grateful for his creative and useful scientific comments and the language correction. We are also very grateful for the useful comments and suggestions of the anonymus reviewer(s).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balogh, P., Katz, S. & Kiss, A.L. The Role of Endocytic Pathways in TGF-β Signaling. Pathol. Oncol. Res. 19, 141–148 (2013). https://doi.org/10.1007/s12253-012-9595-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-012-9595-8