Abstract
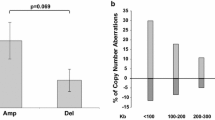
Papillary thyroid carcinoma (PTC) is the most common well-differentiated thyroid cancer. Although the great majority of the cases exhibit an indolent clinical course, some of them develop local invasion with distant metastasis, and a few cases transform into undifferentiated/anaplastic thyroid carcinoma with a rapidly lethal course. To identify gene copy number alterations predictive of metastatic potential or aggressive transformation, array-based comparative genomic hybridization (CGH-array) was performed in 43 PTC cases. Formalin-fixed and paraffin-embedded samples from primary tumours of 16 cases without metastasis, 14 cases with only regional lymph node metastasis, and 13 cases with distant metastasis, recurrence or extrathyroid extension were analysed. The CGH-array and confirmatory quantitative real-time PCR results identified the deletion of the EIF4EBP3 and TRAK2 gene loci, while amplification of thymosin beta 10 (TB10) and Tre-2 oncogene regions were observed as general markers for PTC. Although there have been several studies implicating TB10 as a specific marker based on gene expression data, our study is the first to report on genomic amplification. Although no significant difference could be detected between the good and bad prognosis cases in the A-kinase anchor protein 13 (AKAP13) gene region, it was discriminative markers for metastasis. Amplification in the AKAP13 region was demonstrated in 42.9% and 15.4% of the cases with local or with distant metastasis, respectively, while no amplification was detected in non-metastatic cases. AKAP13 and TB10 regions may represent potential new genomic markers for PTC and cancer progression.
Similar content being viewed by others
Abbreviations
- AKAP13:
-
A-kinase anchor protein 13
- BPTC:
-
Papillary thyroid carcinoma with a bad disease outcome
- CGH-array:
-
Array-based comparative genomic hybridization
- DOP-PCR:
-
Degenerate oligonucleotide-primed PCR
- EIF4EBP3:
-
Eukaryotic initiation factor 4E-binding protein 3
- FGF7:
-
Fibroblast growth factor 7
- GPTC:
-
Papillary thyroid carcinoma with a good disease outcome
- GPTC1:
-
Papillary thyroid carcinoma with a good disease outcome without metastasis
- GPTC2:
-
Papillary thyroid carcinoma with a good disease outcome with regional lymph node metastasis
- LOH:
-
Loss of heterozygosity
- PTC:
-
Papillary thyroid carcinoma
- QRT-PCR:
-
Quantitative real-time polymerase chain reaction
- TB10:
-
Thymosin beta 10
- TRAK2:
-
Trafficking protein, kinesin binding 2
- UTC:
-
Undifferentiated thyroid carcinoma
References
Hundahl SA, Fleming ID, Fremgen AM et al (1998) A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 83:2638–2646
Samaan NA, Schultz PN, Hickey RC et al (1992) The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 75:714–720
Mazzaferri EL, Jhiang SM (1994) Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97:418–428
Gilliland FD, Hunt WC, Morris DM et al (1997) Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer 79:564–573
Sherman SI, Brierley JD, Sperling M et al (1998) Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer 83:1012–1021
Tsang RW, Brierley JD, Simpson WJ et al (1998) The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer 82:375–388
Nikiforova MN, Kimura ET, Gandhi M et al (2003) BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 88:5399–5404
Fagin JA (2004) Challenging dogma in thyroid cancer molecular genetics-role of RET/PTC and BRAF in tumour initiation. J Clin Endocrinol Metab 89:4264–4266
Xing M (2005) BRAF mutation in thyroid cancer. Endocr Relat Cancer 12:245–262
Xing M, Westra WH, Tufano RP et al (2005) BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 90:6373–6379
Kondo T, Ezzat S, Asa SL (2006) Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 6:292–306
Adeniran AJ, Zhu Z, Gandhi M et al (2006) Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol 30:216–222
Giannini R, Ugolini C, Lupi C et al (2007) The heterogeneous distribution of BRAF mutation supports the independent clonal origin of distinct tumour foci in multifocal papillary thyroid carcinoma. J Clin Endocrinol Metab 92:3511–3516
Ciampi R, Nikiforov YE (2007) RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology 148:936–941
Suarez HG (1998) Genetic alterations in human epithelial thyroid tumours. Clin Endocrinol (Oxf) 8:531–546
Garcia-Rostan G, Zhao H, Camp RL et al (2003) Ras mutations are associated with aggressive tumour phenotypes and poor prognosis in thyroid cancer. J Clin Oncol 1:3226–3235
Ciampi R, Knauf JA, Kerler R et al (2005) Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest 115:94–101
Santoro M, Papotti M, Chiappetta G et al (2002) RET activation and clinicopathologic features in poorly differentiated thyroid tumours. J Clin Endocrinol Metab 87:370–379
Tallini G, Santoro M, Helie M et al (1998) RET/PTC oncogene activation defines a subset of papillary thyroid carcinomas lacking evidence of progression to poorly differentiated or undifferentiated tumour phenotypes. Clin Cancer Res 4:287–294
Nikiforov YE (2002) RET/PTC rearrangement in thyroid tumors. Endocr Pathol 13:3–16
Greco A, Miranda C, Pagliardini S et al (1997) Chromosome 1 rearrangements involving the genes TPR and NTRK1 produce structurally different thyroid-specific TRK oncogenes. Genes Chromosomes Cancer 19:112–123
Beimfohr C, Klugbauer S, Demidchik EP et al (1999) NTRK1 re-arrangement in papillary thyroid carcinomas of children after the Chernobyl reactor accident. Int J Cancer 80:842–847
Ito T, Seyama T, Mizuno T et al (1992) Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res 52:1369–1371
Fagin JA, Matsuo K, Karmakar A et al (1993) High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest 91:179–184
Asakawa H, Kobayashi T (2002) Multistep carcinogenesis in anaplastic thyroid carcinoma: a case report. Pathology 34:94–97
Garcia-Rostan G, Camp RL, Herrero A et al (2001) Beta-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Pathol 158:987–996
Huang Y, Prasad M, Lemon WJ et al (2001) Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci USA 98:15044–15049
Puskas LG, Juhasz F, Zarva A et al (2005) Gene profiling identifies genes specific for well-differentiated epithelial thyroid tumors. Cell Mol Biol (Noisy-le-grand) 51:177–186
Puskas LG, Farid NR (2004) Gene expression in thyroid tumors. Cancer Treat Res 122:265–271
Gunn SR, Robetorye RS, Mohammed MS et al (2007) Comparative genomic hybridization arrays in clinical pathology: progress and challenges. Mol Diagn Ther 11:73–77
Hemmer S, Wasenius VM, Knuutila S et al (1999) DNA copy number changes in thyroid carcinoma. Am J Pathol 154:1539–1547
Kjellman P, Lagercrantz S, Hoog A et al (2001) Gain of 1q and loss of 9q21.3-q32 are associated with a less favorable prognosis in papillary thyroid carcinoma. Genes Chromosomes Cancer 32:43–49
Rodrigues R, Roque L, Espadinha C et al (2007) Comparative genomic hybridization, BRAF, RAS, RET, and oligo-array analysis in aneuploid papillary thyroid carcinomas. Oncol Rep 18:917–926
Lee JJ, Au AY, Foukakis T et al (2008) Array-CGH identifies cyclin D1 and UBCH10 amplicons in anaplastic thyroid carcinoma. Endocr Relat Cancer 15:801–815
Finn S, Smyth P, O’Regan E et al (2007) Low-level genomic instability is a feature of papillary thyroid carcinoma: an array comparative genomic hybridization study of laser capture microdissected papillary thyroid carcinoma tumors and clonal cell lines. Arch Pathol Lab Med 131:65–73
Wreesmann VB, Sieczka EM, Socci ND et al (2004) Genome-wide profiling of papillary thyroid cancer identifies MUC1 as an independent prognostic marker. Cancer Res 64:3780–3789
Unger K, Malisch E, Thomas G et al (2008) Array CGH demonstrates characteristic aberration signatures in human papillary thyroid carcinomas governed by RET/PTC. Oncogene 27:4592–4602
Kitamura Y, Shimizu K, Tanaka S et al (2000) Association of allelic loss on 1q, 4p, 7q, 9p, 9q, and 16q with postoperative death in papillary thyroid carcinoma. Clin Cancer Res 6:1819–1825
Pacini F, Schlumberger M, Harmer C et al (2005) Post-surgical use of radioiodine 131I in patients with papillary and follicular thyroid cancer and the issue of remnant ablation: a consensus report. Eur J Endocrinol 153:651–659
Pacini F, Schlumberger M, Dralle G et al (2006) European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 154:767–803
Feher LZ, Balazs M, Kelemen JZ et al (2006) Improved DOP-PCR-based representational whole-genome amplification using quantitative real-time PCR. Diagn Mol Pathol 15:43–48
Telenius H, Carter NP, Bebb CE et al (1992) Degenerate oligonucleotide-primed PCR: general amplification of target DNA by a single degenerate primer. Genomics 13:718–725
Nagy J, Feher LZ, Sonkodi I et al (2005) A second field metachronous Merkel cell carcinoma of the lip and the palatine tonsil confirmed by microarray-based comparative genomic hybridisation. Virchows Arch 446:278–286
Yang YH, Dudoit S, Luu P et al (2002) Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30:e15
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Onno M, Nakamura T, Mariage-Samson R et al (1993) Human TRE17 oncogene is generated from a family of homologous polymorphic sequences by single-base changes. DNA Cell Biol 12:107–118
Martinu L, Masuda-Robens JM, Robertson SE et al (2004) The TBC (Tre-2/Bub2/Cdc16) domain protein TRE17 regulates plasma membrane-endosomal trafficking through activation of Arf6. Mol Cell Biol 24:9752–9762
Masuda-Robens JM, Kutney SN, Qi H et al (2003) The TRE17 oncogene encodes a component of a novel effector pathway for Rho GTPases Cdc42 and Rac1 and stimulates actin remodeling. Mol Cell Biol 23:2151–2161
Smith MJ, Pozo K, Brickley K et al (2006) Mapping the GRIF-1 binding domain of the kinesin, KIF5C, substantiates a role for GRIF-1 as an adaptor protein in the anterograde trafficking of cargoes. J Biol Chem 281:27216–27228
Brickley K, Smith MJ, Beck M et al (2005) GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J Biol Chem 280:14723–14732
Yasuhara T, Okamoto A, Kitagawa T et al (2005) FGF7-like gene is associated with pericentric inversion of chromosome 9, and FGF7 is involved in the development of ovarian cancer. Int J Oncol 26:1209–1216
Hishikawa Y, Tamaru N, Ejima K et al (2004) Expression of keratinocyte growth factor and its receptor in human breast cancer: its inhibitory role in the induction of apoptosis possibly through the overexpression of Bcl-2. Arch Histol Cytol 67:455–464
Firth JD, Putnins EE (2004) Keratinocyte growth factor 1 inhibits wound edge epithelial cell apoptosis in vitro. J Invest Dermatol 122:222–231
Diviani D, Abuin L, Cotecchia S et al (2004) Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J 23:2811–2820
Shibolet O, Giallourakis C, Rosenberg I et al (2007) AKAP13, a RhoA GTPase-specific guanine exchange factor, is a novel regulator of TLR2 signaling. J Biol Chem 282:35308–35317
Wirtenberger M, Tchatchou S, Hemminki K et al (2006) Association of genetic variants in the Rho guanine nucleotide exchange factor AKAP13 with familial breast cancer. Carcinogenesis 27:593–598
Chiappetta G, Pentimalli F, Monaco M et al (2004) Thymosin beta-10 gene expression as a possible tool in diagnosis of thyroid neoplasias. Oncol Rep 12:239–243
Takano T, Hasegawa Y, Miyauchi A et al (2002) Quantitative analysis of thymosin beta-10 messenger RNA in thyroid carcinomas. Jpn J Clin Oncol 32:229–232
Hall AK (1995) Thymosin beta-10 accelerates apoptosis. Cell Mol Biol Res 41:167–180
Maelan AE, Rasmussen TK, Larsson LI (2007) Localization of thymosin beta10 in breast cancer cells: relationship to actin cytoskeletal remodeling and cell motility. Histochem Cell Biol 127:109–113
Califano D, Monaco C, Santelli G et al (1998) Thymosin beta-10 gene overexpression correlated with the highly malignant neoplastic phenotype of transformed thyroid cells in vivo and in vitro. Cancer Res 58:823–828
Santelli G, Califano D, Chiappetta G et al (1999) Thymosin beta-10 gene overexpression is a general event in human carcinogenesis. Am J Pathol 155:799–804
Lee SH, Son MJ, Oh SH et al (2005) Thymosin {beta}(10) inhibits angiogenesis and tumour growth by interfering with Ras function. Cancer Res 65:137–148
Santelli G, Bartoli PC, Giuliano A et al (2002) Thymosin beta-10 protein synthesis suppression reduces the growth of human thyroid carcinoma cells in semisolid medium. Thyroid 12:765–772
Huff T, Muller CS, Otto AM et al (2001) beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol 33:205–220
Acknowledgements
This work was supported by grants from the Hungarian Ministry of Health (ETT 400/2003) and a bilateral grant between the Hungarian Prime Minister’s Office and the Hungarian Academy of Sciences (40.232/1/2005), the Economic Development Oriented Project of the Békés County Self-Government and the Berkes Foundation of the Pándy Kálmán County Hospital, Gyula. We would also like to thank the Jósa András County Hospital in Nyíregyháza, the Erzsébet Hospital in Hódmezővásárhely, the County Hospital in Kecskemét, the Pándy Kálmán County Hospital in Gyula, and especially Z. Nemes, Department of Pathology, Medical and Health Science Center, University of Debrecen, Hungary, for providing the thyroid carcinoma samples.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fehér, L.Z., Pocsay, G., Krenács, L. et al. Amplification of Thymosin Beta 10 and AKAP13 Genes in Metastatic and Aggressive Papillary Thyroid Carcinomas. Pathol. Oncol. Res. 18, 449–458 (2012). https://doi.org/10.1007/s12253-011-9467-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-011-9467-7