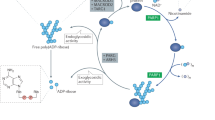
Abstract
Today, the number of cancer patients throughout the world is increasing alarmingly and as per the World Health Organisation (WHO) data and statistics the prediction for the year 2020 will be 15 million new cases as compared to only 10 million cases in year 2000 leaving us dumbfounded. A lot of effort has been put in by researchers and scientists over decades to find drugs helpful in the treatment of cancers for the benefit of patients—The latest being the Poly ADP-ribose polymerase (PARP) and the Poly ADP-ribose glycohydrolase (PARG) inhibitors. This review highlights their mechanism of action under the rationale of their use and current development in the field of cancer.
Similar content being viewed by others
Abbreviations
- PARP:
-
Poly ADP ribose polymerase
- PARG:
-
Poly ADP-ribose glycohydrolase
References
D’amours D, Desnoyers S, D’silva I, Poirier GG (1999) Poly (ADP-ribosyl)ation reactions in the regulation of nuclear functions. J Biochem 342:249–268
Parise RA, Shawaqfeh M, Egorin MJ, Beumer JH (2008) Liquid chromatography-mass spactometric assay for quantitation in human plasma of ABT-888, an orally available, small molecule inhibitor of poly (ADP-ribose) polymerase. J Chromatogr B Analyt Technol Biomed Life Sci 872(1–2):141–147
Hochegger H, Dejsuphong D, Fukushima T, Morrison C et al (2006) Parp-1 protects homologous recombination from interferenve by Ku and Ligase IV in vertebrate cells. EMBO J 25:1305–1314
Virag L, Szabo C (2002) The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol Rev 54:375–429
Meyer RG, Meyer-Ficca ML, Jacobson EL, Jacobson MK (2003) Human poly (ADP-ribose) glycohydrolase (PARG) gene and the common promoter sequence it shares with inner mitochondrial membrane translocase 23 (TIM23). Gene 314:181–190
Koh DW, Lawler AM, Poitras MF et al (2004) Failure to degrade poly (ADP-ribose) increased sensitivity to cytotoxicity and early embryonic lethality. PNAS 101(51):17699–17704
Cortes U, Tong WM, Coyle DL et al (2004) Depletion of the 110-Kilodalton isoform of poly (ADP-ribose) glycohydrolase increases sensitivity to genotoxic and endotoxic stress in mice. MCB 24(16):7163–7178
Poitras MF, Koh DW, Yu SW et al (2007) Spatial and functional relationship between poly (ADP-ribose) polymerase-1 and Poly (ADP-ribose) glycohydrolase in the brain. Neuroscience 148(1):198–211
Miwa M, Matsutani (2007) PolyADP-ribosylation and cancer. Cancer Sci 98(10):1528–1535
Blenn C, Althaus FR, Manlanga M (2006) Poly (ADP-ribose) glycohydrolase silencing protects against H2O2-induced cell death. J Biochem 396:419–429
Ratnam K, Low JA (2007) Current development of clinical poly (ADP-ribose) inhibitor in oncology. Clin Cancer Res 13(5):1383–1388
Decker P, Miranda EA, De Murcia G, Muller S (1999) An improved nonisotopic test to screen a large series of new inhibitor molecules of poly (ADP-ribose) polymerase activity for therapeutic applications. Clin Cancer Res 5:1169–1172
Cuzzocrea S, Paola RD, Mazzon E et al (2005) PARG activity mediates intestinal injury induced by splanchnic artery occlusion and reperfusion. FASEB J 19:558–566
Plummer R, Jones C, Middleton M et al (2008) Phase I study of the poly (ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced Solid Tumors. Clin Cancer Res 14(23):7917–7923
Valenzuela MT, Guerrerro R, Nunez MI et al (2002) PARP-1 modifies the effectiveness of P53 mediated DNA damage response. Oncogene 21(7):1108–1116
Oei SL, Ziegler M (2000) ATP for the DNA ligation step in base excision repair is generated from poly (ADP-ribose). J Biol Chem 275:23234–23239
McCabe N, Turner NC, Lord CJ et al (2006) Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly (ADP-ribose) polymerase inhibition. Cancer Res 66(16):8109–8115
Eguchi Y, Shimizu S, Tsujimoto Y (1997) Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res 57:1835–1840
Kumari SR, Mendoza-Alvarez H, Alvarez-Gonzalez R (1998) Functional interactions of p53 with poly(ADP-ribose) polymerase (PARP) during apoptosis following DNA damage: covalent poly(ADP-ribosyl)ation of p53by exogenous PARP and noncovalent binding of p53 to the Mr 85,000 proteolytic fragment1. Cancer Res 58:5075–5078
Affar EB, Germain M, Winstall E et al (2001) Caspase-3-mediated processing of poly (ADP-ribose) glycohydrolase during apoptosis. JBC 276(4):2935–2942
Koh DW, Dawson TM, Dawson VL (2005) Mediation of cell death by Poly (ADP-ribose)-1. Pharmacol Res 52(1):5–14
Bhaskara, et al (2009) Differrential PARP cleavage: an indication for existence of multiple forms of cell death in human gilomas. Neurology India 57(3)
Hung Q, Wu YT, Tan HL, Ong CN, Shen HM (2009) A novel function of PARP-1 in modulation of autophagy and necrosis under oxidative stress. Cell Death Diff 16:264–277
Lee Y, Shacter E (1999) Oxidative stress inhibits apoptosis in human lymphoma cells. J Bio Chem 274:19792–19798
Walisser JA, Thies RC (1999) Poly (ADP-ribose) polymerase inhibition in oxidant stressed endothelial cells prevents oncosis and permits caspase activation and apoptosis. Exp Cell Res 251(12):401–413
Mathews MT, Berk BC (2008) PARP-1 inhibition prevents oxidative and nitrosative stress–induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler Thromb Vasc Biol 28:711–717
Kovacs K, Toth A, Deres P et al (2006) Critical role of PI3-kinase/Akt activation in the PARP inhibitor induced heart function recovery during ischemia–reperfusion. Biochem Pharmacol 71(4):441–452
Klaidman L, Morales M, Kem S et al (2003) Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology 69(3):150–157
Filipovic DM, Meng X, Reeves WB (1999) Inhibition of PARP prevents oxidant-induced necrosis but not apoptosis in LLC-PK1 cells. Am J Physiol Renal Physiol 277:428–436
Roesner JP, Vagts DA, Iber T et al (2006) Protective effects of PARP inhibition on liver microcirculation and function after haemorrhagic shock and resuscitation in male rats. Intensive Care Med 32(10):1649–1657
Oku H, Goto W, Tsujimoto M et al (2003) Effects of Poly(ADP-ribose) polymerase (PARP) Inhibitor on NMDA-induced retinal injury. Invest Ophthalmol Vis Sci 44: E-Abstract 27
Charron MJ, Bonner-Weir S (1999) Implicating PARP and NAD+ depletion in type I diabetes. Nat Med 5:269–270
Ha HC, Snyder SH (1999) Poly (ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. PNAS 96:13978–13982
Hanai S, Kanai M, Ohashi S et al (2004) Loss of poly (ADP-ribose) glycohydrolase causes progressive neurodegeneration in Drosophila melanogaster. PNAS 101(1):82–86
Keil C, Gröbe T, Oei SL (2006) MNNG-induced cell death is controlled by interactions between PARP-1, Poly (ADP-ribose) glycohydrolase, and XRCC1. J Biochem 281:34394–34405
Ménissier-de Murcia J, Mark M, Wendling O, Wynshaw-Boris A, de Murcia G (2001) Early embryonic lethality in PARP-1 Atm double-mutant mice suggests a functional synergy in cell proliferation during development. MCB 21(5):1828–1832
Yang YG, Cortes U, Patnaik S, Jasin M, Wang Z (2004) Ablation of PARP-1 does not interfere with the repair of DNA double-strand breaks, but compromises the reactivation of stalled replication forks. Oncogene 23:3872–3882 [abstract]
Nozaki T, Masutani M, Watanabe M et al (1999) Syncytiotrophoblastic giant cells in Teratocarcinoma like tumors derived from Parp-disrupted mouse embryonic stem cells. PNAS 96(23):13345–13350
Saxena A, Saffery R, Wong LH et al (2002) Centromere proteins Cenpa, Cenpb, and Bub3 interact with poly (ADP-ribose) polymerase-1 protein and are poly (ADP-ribosyl) ated. J Biol Chem 277:26921–26926
Bhatia M, Kirkland JB, Meckling-Gill KA (1996) Overexpression of poly (ADP-ribose) polymerase promotes cell cycle arrest and inhibits neutrophilic differentiation of NB4 acute promyelocytic leukemia cells. Cell Growth Differ 7(1):91–100
Bhatia M, Kirkland JB, Meckling-Gill KA (1995) Modulation of poly (ADP-ribose) polymerase during neutrophilic and monocytic differentiation of promyelocytic (NB4) and myelocytic (HL-60) leukaemia cells. J Biochem 308:131–137
Harnacke K, Kruhoffer M, Orntoft TF, Hass R (2005) Down-modulation of poly(ADP-ribose) polymerase-1 (PARP-1) in human TUR leukemia cells restores transcriptional responsiveness for differentiation and cell cycle arrest. Eur J Cell Biol 84(11):885–896 [abstract]
Masutani M, Nozaki T, Watanabe M et al (2001) Involvement of poly(ADP-ribose) polymerase in trophoblastic cell differentiation during tumorigenesis. Mutant Res 477:111–117
Shiokawa M, Masutani M, Fujihara H et al (2005) Genetic alteration of poly(ADP-ribose) polymerase-1 in human germ cell tumors. Jpn J Clin Oncol 35(2):97–102
Sevigny MB, Silva JM, Lan WC, Alano CC, Swanson RA (2003) Expression and activity of poly(ADP-ribose) glycohydrolase in cultured astrocytes, neurons, and C6 glioma cells. Mol Brain Res 117(2):213–220 [abstract]
Kraus W, Lis J (2003) PARP goes transcription. Cell 113(6):677–683
Pavri R, Lewis B, Kim TK et al (2005) PARP-1 determines specificity in a retinoid signaling pathway via direct modulation of mediator. Moll Cell 18(1):83–96 [abstract]
Idogawa M, Yamada T, Honda K et al (2005) Poly(ADP-ribose) polymerase-1 is a component of the oncogenic T-cell factor-4/beta-catenin complex. Gastroenterology 128(7):1919–1936 [abstract]
Soldatenkov VA, Albor A, Patel BKR et al (1999) Regulation of the human poly(ADP-ribose) polymerase promoter by ETS transcription factor. Oncogene 18(27):3954–3962
Ogino H, Nozaki T, Gunji A et al (2007) Loss of Parp-1 affects gene expression profile in a genome-wide manner in ES cells and liver cells. BMC Genomics 8:227. doi:10.1186/1471-2164-8-227
Aldinussi A, Gerlini G, Fossati S et al (2007) A key role for poly (ADP-ribose)-1 during human dendritic cell maturation. J Immuno 179:305–312
Zaniolo K, Desnoyers S, Leclerc S, Guérin S (2007) Regulation of poly(ADP-ribose) polymerase-1 (PARP-1) gene expression through the post-translational modification of Sp1: a nuclear target protein of PARP-1. BMC Genomics 8:96. doi:10.1186/1471-2199-8-96
Tong WM, Hande MP, Lansdorp PM, Wang ZQ (2001) DNA strand break-sensing molecule poly(ADP-Ribose) polymerase cooperates with p53 in telomere function, chromosome stability, and tumor suppression. Mol Cell Biol 21(12):4046–4054
Eberhart CG (2003) Medulloblastoma in mice lacking p53 and PARP. Am J Pathol 162(1):7–10
Oei SL, Griesenbeck J, Schweiger M, Ziegler M (1998) Regulation of RNA polymerase II-dependent transcription by poly(ADP-ribosyl)ation of transcription factors. JBC 273:31644–31647
Hassa PO, Buerki C, Lombardi C, Imhof R, Hottiger MO (2003) Transcriptional coactivation of nuclear factor Kappa B-dependent gene expression by p300 is regulated by poly (ADP)-ribose polymerase-1. JBC 278(46):45145–45153
Nie J, Sakamoto S, Song D, Qu Z, Ota K, Taniguchi T (1998) Interaction of Oct-1 and automodification domain of poly (ADP-ribose) synthetase. FEBS Lett 424(1–2):27–32
Dear TN, Hainzl T, Follo M (1997) Identification of interaction partners for the basic-helix-loop-helix protein E47. Oncogene 14:891–898
Butler AJ, Ordhal CP (1999) Poly (ADP-Ribose) polymerase binds with transcription enhancer factor 1 to MCAT1 elements to regulate muscle-specific transcription. Mol Cell Boil 19(1):296–306
Taniguchi T, Agemori M, Kameshita I, Nishikimi M, Shizuta Y (1982) Participation of poly(ADP-ribosyl)ation in the depression of RNA synthesis caused by treatment of mouse lymphoma cells with methylnitrosourea. J BC 257:4027–4030
Shimokawa T, Masutani M, Nagasawa S et al (1999) Isolation and cloning of rat poly (ADP-ribose) glycohydrolase: presence of a potential nuclear export signal conserved in mammalian orthologs. J Biochem 126:748–755
Erdelyi K, Kiss A, Bankondi E et al (2005) Gallotannin inhibits the expression of chemokines and inflammatory cytokines in A549 cells. Mol Pharmacol 68:895–904
Frizzell KM, Gamble MJ, Berrocal JG (2009) Global analysis of transcriptional regulation by poly(ADP-ribose) polymerase-1 and poly(ADP-ribose) glycohydrolase in MCF-7 human breast cancer cells. J Biol Chem 284(49):33926–33938 [abstract]
Wang ZQ, Stingl L, Morrison C et al (1997) PARP is important for genomic stability but dispensable in? Apoptosis. Genes Dev 11:2347–2358
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29:15–18
Tentori L, Lacal PM, Muzi A et al (2007) Poly(ADP-ribose) polymerase (PARP) inhibition or PARP-1 gene deletion reduces angiogenesis. Eur J Cancer 43(14):2124–2133
Pyriochou A, Olah G, Deitch EA, Szabo C, Papapetropoulos A (2008) Inhibition of angiogenesis by the poly (ADP-ribose) polymerase inhibitor PJ-34. IJMM 22:113–118
Rajesh M, Mukhopadhyay P, Bátkai S et al (2006) Pharmacological inhibition of poly (ADP-ribose) polymerase inhibits angiogenesis. BBRC 350(2):352–357
Rajesh M, Mukhopadhyay P, Godlewski G (2006) Poly (ADP-ribose) polymerase inhibition decreases angiogenesis. BBRC 350(4):1056–1062
Berger NA, Adams JW, Sikorski GW et al (1978) Synthesis of DNA and poly (Adenosine Diphosphate Ribose) in normal and chronic lymphocytic leukemia lymphocytes. J Clin Inves 62:111–118
Wielckens K, Garbrecht M, Kittler M, Hilz H (1980) ADP-ribosylation of nuclear proteins in normal lymphocytes and in low- grade malignant non-Hodgkin lymphoma cells. Eur J Biochem 104:279–287
Hirai K, Ueda K, Hayaishi O (1983) Abberation of poly (adenosine diphosphate ribose) in human colon adenomatous polyps and cancers. Cancer Res 43:3441–3446
Fukushima M, Kuzuya OK, Ikai K (1981) Poly (ADP-ribose) synthesis in human cervical cancer cell diagnostic cytological usefulness. Cancer Lett 14:227–236
Shiobara M, Miyazaki M, Ito H et al (2001) Enhanced polyadenosine diphosphate ribosylation in cirrhotic liver and carcinoma tissues in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 16:338–344
Lin L, Li J, Wang YL et al (2009) Relationship of PARG with colorectal carcinoma PARP, VEGF and b-FGF in colorectal carcinoma. Chin J Cancer Res 21(2):135–141
Leal AP et al (2009) PARP inhibitors: New partners in the therapy of cancer and inflammatory diseases. Free Radic Biol Med (in press). Doi:10.1016
Lord CJ, McDonald S, Swift S et al (2008) A high-throughput RNA interference screen for DNA repair determinants of PARP inhibitor sensitivity. DNA Repair (Amst) 7:2010–2019
Turner NC, Lord CJ, Iorns E et al (2008) A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J 27(9):1368–1377
Ashworth A (2008) A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 26(22):3785–3790
Bryant HE, Schultz N, Thomas HD et al (2005) Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434:913–917 [abstract]
Turner N, Tutt A, Ashworth A (2005) Targeting the DNA repair defect of BRCA tumours. Curr Opin Pharmacol 5(4):388–393
De Soto JA, Wang X, Tominaga Y et al (2006) The inhibition and treatment of breast cancer with poly (ADP-ribose) polymerase (PARP-1) inhibitors. Int J Biol Sci 2(4):179–185
De Soto JA, Deng CX (2006) PARP-1 inhibitors: are they the long-sought genetically specific drugs for BRCA1/2-associated breast cancers? Int J Med Sci 3(4):117–123
Curtin NJ (2005) PARP inhibitors for cancer therapy. Expert Rev Mol Med 7(4):1–20 [abstract]
Liu X, Shi Y, Guan R et al (2008) Potentiation of temozolomide cytotoxicity by poly(ADP)ribose polymerase inhibitor ABT-888 requires a conversion of single stranded DNA damages to double-stranded DNA breaks. Mol Cancer Res 6(10):1621–1629
Suesse S, Scholz CJ, Buerkle A, Iler LW (2004) Poly (ADP-ribose) polymerase (PARP-1) and P53 independently function in regulating double strand break repair in primate cells. Nucleic Acids Res 32:2669–2680
Purnell MR, Whish WJD (1980) Novel inhibitors of poly (ADP-ribose) synthetase. J Biochem 185:775–777
Banasik M, Komura H, Shimoyama M, Ueda K (1992) Specific inhibitors of poly (ADP-ribose) synthetase and mono(ADP-ribosyl) transferase. J Biol Chem 267:1569–1575
Genovese T, Di Paola R, Catalano P et al (2004) Treatment with a novel poly(ADP-ribose) glycohydrolase inhibitor reduces development of septic shock-like syndrome induced by zymosan in mice. Crit Care Med 32(6):1365–1374
Masutani M, Shimokawa T, Igarashi M et al (2002)Inhibition of poly(ADP-ribose) glycohydrolase activity by cyclic peptide antibiotics containing piperazic acid residues. Proceedings of the Japanese academy; Ser B, physical and biological sciences 78(1):15–17
Formentini L, Arapistas P, Pitelli M et al (2008) Mono-galloyl glucose derivatives are potent poly(ADP-ribose) glycohydrolase (PARG) inhibitors and partially reduced PARP-1 dependent cell death. BJP 155:1235–1249
Yang X, Lippman ME (1999) BRCA1 and BRCA2 in breast cancer. Breast Cancer Res Treat 54(1):1–10
De Soto JA, Wang X, Tominaga Y et al (2006) The inhibition and treatment of breast cancer with poly (ADP-ribose) polymerase (PARP-1) inhibitors. Int J Biol Sci 2(4):179–185
Munozo-Gamez JA, Martin-Olivia D, Aguilar-Quesada R (2005) PARP inhibition sensitizes p53-deficient breast cancer cells to doxorubicin-induced apoptosis. Biochem J 386:119–125
Rottenberg S, Jaspers JE, Kersbergen A et al (2008) High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. PNAS 105(44):17079–17084
Gopall R, Hong C & Yi S (2009) Gefitinib as monotherapy in the first-line setting in non-small cell lung Cancer. http://www.ispub.com/journal/the_internet_journal_of_pulmonary_medicine/volume_11_number_2_5/article/gefitinib-as-monotherapy-in-the-first-line-setting-in-non-small-cell-lung-cancer.html
Albert JM, Cao C, Kim KW et al (2007) Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res 13(10):3033–3042
Kinders RJ, Hollingshead M, Khin S et al (2008) Preclinical modeling of a phase 0 clinical trial: qualification of a pharmacodynamic assay of poly (ADP-ribose) polymerase in tumor biopsies of mouse xenografts. Clin Cancer Res 14(21):6877–6885
Donawho CK, Luo Y, Penning TD et al (2007) ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 13(9):2728–2737
Calabrese CR, Batey MA, Thomas HD (2003) Identification of potent nontoxic poly (ADP-ribose) polymerase-1 inhibitors: chemopotentiation and pharmacological studies. Clin Cancer Res 9:2711–2718
Hao LX, Wang YL, Cai L, YY LI (2007) Inhibitory effect of 5-Aminoisoquinolinone on Parp activity in colon carcinoma cell line HT-29. Chin J Cancer 26(6):566–571
Li M, Threadgill MD, Wang YL, Cai L, Lin X (2009) A poly (ADP-ribose) polymerase inhibition down-regulates expression of metastasis-related genes in CT26 colon carcinoma cells. Pathobiology 76:108–116
Tentori L, Portarena I, Barbarino M et al (2003) Inhibition of telomerase increases resistance of melanoma cells to temozolomide, but not to temozolomide combined with poly (ADP-ribose) polymerase inhibitor. Mol Pharmacol 63:192–202
Tentori L, Leonetti C, Scarsella M et al (2003) Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin Cancer Res 9:5370–5379
Tentori L, Leonetti C, Scarsella M et al (2005) Poly(ADP-ribose) glycohydrolase inhibitor as chemosensitiser of malignant melanoma for temozolomide. Eur J Cancer 41(18):2948–2957
Dungey FA, Caldecott KW, Chalmers AJ (2009) Enhanced radiosensitisation of human glioma cells by combining inhibition of PARP with inhibition of Hsp90. Mol Cancer Ther 8(8):2243–2254
Fong PC, Boss DS, Yap TA et al (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361:123–134
Gien LT, Mackay HJ (2010) The emerging role of PARP inhibitors in the treatment of epithelial ovarian cancer. J Oncol. doi:10.1155/2010/151750
Grants support
This work was supported by the National Nature Science Foundation of China (NSFC: 30870946)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fauzee, N.J.S., Pan, J. & Wang, Yl. PARP and PARG Inhibitors—New Therapeutic Targets in Cancer Treatment. Pathol. Oncol. Res. 16, 469–478 (2010). https://doi.org/10.1007/s12253-010-9266-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-010-9266-6