Abstract
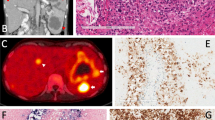
Among the 300 peripheral T-cell lymphomas (PTCL) searched for EBV positive non-resting B-cells by EBER in situ hybridization 12 have been identified with various forms of EBV-driven B-cell proliferation. This could be categorized into three major forms. i. In the first form scattered immature, mononuclear B-cells of immuno-, centroblastic type with CD20+. CD30+ CD45+, LMP1+ phenotype, reactive appearance and polyclonal immunoglobulin heavy chains gene rearrangement (IgH-R) were admixed to the PTCL cells. ii. The second form mimicked diffuse large B-cell lymphoma as homogenous sheets, largely demarcated from the PTCL, of mononuclear, immature B-cell of CD20+, CD30+, CD45+, LMP1+, EBNA-2+ phenotype but with lack of monoclonal IgH-R were present. iii. In the third form scattered Hodgkin-Reed-Sternberg (HRS) type of cells were noticed which exhibited the CD15+/−, CD20−/+, CD30+, CD45−, LMP1+, EBNA-2- phenotype and in 50% showed clonal IgH gene rearrangement in whole tissue DNA extract. The IgH associated transcription factors’ (OCT2, BOB.1/OBF.1, PU.1) expression patterns in these cells corresponded to those of HRS cells in cHL. Based on analysis of 65 PTCLs, we have identified in the positive cases a highly significant increase of EBV+ small, reactive, resting B-cell compartment (75.9 / 100 HPF in PTCL vs. 1.5 / 100 HPF in control lymph nodes) likely to be due to the decreased immune surveillance. This progressive accumulation of EBV+ by-stander B-cell population in PTCLs might be the source of various B-cell proliferations, which in any form represent major diagnostic pitfalls and require a careful differential diagnostic procedure.

Similar content being viewed by others
Abbreviations
- CDR:
-
complementary determining region
- CHL:
-
classical Hodgkin lymphoma
- DLBCL:
-
diffuse large B-cell lymphoma
- EBER:
-
Epstein Barr early response gene
- EBNA:
-
Epstein Barr nuclear antigen
- EBV:
-
Epstein Barr virus
- FR:
-
framework region
- HPF:
-
high power field
- HRS:
-
Hodgkin Reed Sternberg
- IgH-R:
-
immunoglobulin heavy chain gene rearrangement
- LBC:
-
large B-cell
- LBCR-TCL:
-
large B-cell rich T-cell lymphoma
- LMP-1:
-
latent membrane protein-1
- NOS:
-
not otherwise specified
- PCR:
-
polymerase chain reaction
- PTCL:
-
peripheral T-cell lymphoma
- PTCL-LB:
-
PTCL with proliferation of large B-cells
- TCR-BCL:
-
T-cell rich B-cell lymphoma
- TCR-γ-R:
-
T-cell receptor gamma gene rearrangement
- TF:
-
transcription factor
- TL:
-
T-cell lymphoma
References
Barry ST, Jaffe SE, Sorbara L et al (2003) Peripheral T-cell lymphomas expressing CD30 and CD15. Am J Surg Pathol 27:1513–1521
Quintanilla-Martinez L, Fend F, Rodriguez Moguel L et al (1999) Peripheral T-cell lymphoma with Reed-Sternberg-like cell of B-cell phenotype and genotype associated with Epstein-Barr virus infection. Am J Surg Pathol 23:1233–1240
Pajor L, Kajtár B, Jáksó P et al (2006) Epstein-Barr virus-induced B-cell proliferation of Hodgkin’s and Reed-Sternberg cell pheno- and genotype may develop in peripheral T-cell lymphomas. Histopathology 49:553–557
Ho WYJ, Ho CSF, Chan CLA et al (1998) Frequent detection of Epstein-Barr virus-infected B cells in peripheral T-cell lymphomas. J Pathol 185:79–85
Niedobitek G, Baumann I, Brabletz T et al (2000) Hodgkin’s disease and peripheral T-cell lymphoma: composite lymphoma with evidence of Epstein-Barr virus infection. J Pathol 191:394–399
Zettl A, Lee S-S, Rüdiger T et al (2002) Epstein-Barr virus-associated B-cell lymphoproliferative disorders in angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J Clin Pathol 117:368–379
Tornoczky T, Kelényi G, Pajor L et al (1998) EBER oligonucleotide RNA in situ hybridization in EBV associated neoplasms. Patol Oncol Res 4:201–205
Trainor KJ, Brisco MJ, Wan JH et al (1991) Gene rearrangement in B- and T-lymphoproliferative disease detected by the polymerase chain reaction. Blood 78:192–196
Pajor L, Matolcsy A, Vass JA et al (1998) Phenotypic and genotypic analyses of blastic cell population suggest that pure B-lymphoblastic leukaemia may arise from myelodysplastic syndrome. Leuk Res 22:13–17
Higgins PTJ, Rijn M, Jones DC et al (2000) Peripheral T-cell lymphoma complicated by a proliferation of large B cells. Am J Clin Pathol 114:236–247
Reichard KK, Schwartz JE, Higgins PJ et al (2006) CD10 expression in peripheral T-cell lymphomas complicated by a proliferation of large B-cells. Modern Pathol 19:337–343
Thorley-Lawson AD, Gross A (2004) Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 350:1328–1337
Klein E (2004) Non-proliferative interactions of Epstein-Barr virus and human B lymphocytes. Fiola Biol-Prague 50:131–135
Amon W, Farrell JP (2005) Reactivation of Epstein-Barr virus from latency. Rev Med Virol 15:149–156
Atayar C, Poppema S, Blokzijl T et al (2005) Expression of the T-cell transciption factors, GATA-3 and T-bet, in the neoplastic cells of Hodgkin lymphomas. Am J Pathol 166:127–134
McCune CR, Syrbu IS, Vasef AM (2006) Expression profiling of transcription factors Pax-5, Oct-1, Oct-2, BOB.1, and PU.1 in Hodgkin’s and non-Hodgkin’s lymphomas: a comparative study using high throughput tissue microarrays. Modern Pathol 19:1010–1018
Korbjuhn P, Anagnostopoulos I, Hummel M et al (1993) Frequent latent Epstein-Barr virus infection of neoplastic T cells and Bystander B cells in human immunodeficiency virus-negative European peripheral pleomorphic T-cell lymphomas. Blood 82:217–223
Suwiwat S, Pradutkanchana J, Ishida T et al (2007) Quantitative analysis of cell-free Epstein-Barr virus DNA in the plasma of patients with peripheral T-cell and NK-cell lymphomas and peripheral T-cell proliferative diseases. J Clin Virol 40:277–283
Acknowledgement
This work was supported by the grant 268/2006 sponsored by the Hungarian Scientific Council of Health (ETT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smuk, G., Illés, Á., Keresztes, K. et al. Pheno- and Genotypic Features of Epstein-Barr Virus Associated B-Cell Lymphoproliferations in Peripheral T-Cell Lymphomas. Pathol. Oncol. Res. 16, 377–383 (2010). https://doi.org/10.1007/s12253-009-9233-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-009-9233-2