Abstract
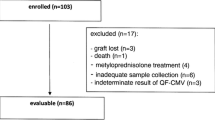
Cytomegalovirus (CMV) has been recognized as the most important viral pathogen in persons undergoing bone marrow transplantation (BMT). The aim was to develop a quantitative PCR assay to quantify CMV DNA in peripheral blood leukocytes (PBLs) of bone marrow transplantation (BMT) patients. An in-house real-time PCR assay based on TaqMan technology was developed to monitor the quantity of CMV DNA in PBLs of the BMT recipients. Sequential blood samples (415 specimens) were collected from 43 patients as weekly intervals until day 100 after transplantation. The CMV DNA was quantified in parallel with the pp65 antigenemia assay in PBL samples. Viral reactivation occurred in 51% and 41.8% of the recipients as detected by RQ-PCR and antigenemia assays respectively. There was a significant correlation between both assays (P < 0.0001); however, the RQ-PCR was more sensitive than the antigenemia. CMV DNA was detected by the RQ-PCR by a median of 14 days earlier than the antigenemia. Preemptive therapy was implemented in the antigenemia positive cases. The administration of ganciclovir led to a rapid decrease in the viral load. After preemptive therapy, the antigenemia achieved a negative result earlier than the RQ-PCR assay (a median of 17.5 days). An increase of viral load in both quantitative assays and of cyclosporine serum level were identified as the most significant risk factors for CMV reactivation. The quantitative CMV PCR might be a useful tool for monitoring the CMV reactivation and guiding the efficacy of the CMV preemptive therapy in BMT recipients.





Similar content being viewed by others
References
Boeckh M, Boivin G (1998) Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin Microbiol Rev 11:533–554
Salzberger B, Bowden RA, Hackman R et al (1997) Neutropenia in allogeneic marrow transplant recipients receiving ganciclovirfor prevention of CMV disease: risk factors and outcome. Blood 90:2502–2508
Li CR, Greenberg PD, Gilbert MJ et al (1994) Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood 83:1971–1979
Goodrich JM, Bowden RA, Fisher L et al (1993) Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med 118:173–178
Winston DJ, Ho WG, Bartoni K et al (1999) Ganciclovir prophylaxis of cytomegalovirus infection and disease in allogeneic bone marrow transplant recipients. Results of a placebo-controlled, double-blind trial. Ann Intern Med 118:179–184
Goodrich JM, Mori M, Gleaves CA et al (1991) Early treatment with ganciclovir to prevent cytomegalovirus disease after allogeneic bone marrow transplantation. N Engl J Med 325:1601–1607
Schmidt GM, Horak DA, Niland JC et al (1991) A randomized controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants. N Engl J Med 324:1005–1011
Aitken CW, Barrett-Muir C, Millar K et al (1999) Use of molecular assays in diagnosis and monitoring of cytomegalovirus disease following renal transplantation. J Clin Microbiol 37:2804–2807
Griscelli F, Barrois M, Chauvin S et al (2001) Quantification of human cytomegalovirus DNA in bone marrow transplant recipients by real-time PCR. J Clin Microbiol 39:4362–4369
Gleaves CA, Smith TH, Shuster EA et al (1984) Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol 19:917–919
Boeckh M, Gooley TA, Myerson D et al (1996) Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood 10:4063–4071
Hebart H, Gamer D, Loeffer J et al (1998) Evaluation of Murex CMV DNA hybrid capture assay for the detection and quantification of cytomegalovirus infection in patients following allogeneic stem cell transplantation. J Clin Microbiol 36:1333–1337
Einsele H, Steidle M, Vallbracht A et al (1991) Early occurrence of human cytomegalovirus infection after bone marrow transplantation as demonstrated by the polymerase chain reaction technique. Blood 77:1104–1110
Gault E, Miche Y, Dehe’e A et al (2001) Quantification of human cytomegalovirus DNA by real-time PCR. J Clin Microbiol 39:772–775
Machida U, Kami M, Fukui T et al (2000) Real-time automated PCR for early diagnosis and monitoring of cytomegalovirus infection after bone marrow transplantation. J Clin Microbiol 38:2536–2542
Mori T, Okamoto S, Matsuoka S et al (2000) Risk-adapted preemptive therapy for cytomegalovirus disease in patients undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant 25:765–769
Nitsche A, Steuer N, Schmidt CA et al (2000) Detection of human cytomegalovirus DNA by real-time quantitative PCR. J Clin Microbiol 38:2734–2737
Razonable RR, Brown RA, Espy MJ et al (2001) Comparative quantification of cytomegalovirus (CMV) DNA in solid-organ transplant recipients with CMV infection by using two high-throughput automated systems. J Clin Microbiol 39:4472–4476
Schaade L, Kockelkorn P, Ritter K et al (2000) Detection of cytomegalovirus DNA in human specimens by lightCycler PCR. J Clin Microbiol 38:4006–4009
Tanaka N, Kimura H, Iida K et al (2000) Quantitative analysis of cytomegalovirus load using a real-time PCR assay. J Med Virol 60:455–462
Yun Z, Lewensohn-Fuchs I, Ljungman P et al (2000) Real-time monitoring of cytomegalovirus infections after stem cell transplantation using the TaqMan polymerase chain reaction assays. Transplantation 69:1733–1736
Yakushiji K, Gondo H, Kamezaki K et al (2002) Monitoring of cytomegalovirus reactivation after allogeneic stem cell transplantation: comparison of an antigenemia assay and quantitative real-time polymerase chain reaction. Bone Marrow Transplant 29:599–606
Leruez-Ville M, Ouachee M, Delarue R et al (2003) Monitoring cytomegalovirus infection in adult and pediatric bone marrow transplant recipients by a real-time PCR assay performed with blood plasma. J Clin Microbiol 41:2040–2046
Tanabe K, Tokumoto T, Ishikawa N et al (1997) Comparative study of cytomegalovirus (CMV) antigenemia assay, polymerase chain reaction, serology, and shell vial assay in the early diagnosis and monitoring of CMV infection after renal transplantation. Transplantation 64:1721–1725
Seropian S, Ferguson D, Salloum E et al (1998) Lack of reactivity to CMV pp65 antigenemia testing in a patient with CMV disease following allogeneic bone marrow transplant. Bone Marrow Transplant 22:507–509
Solano C, Munoz I, Gutierrez A et al (2001) Qualitative plasma PCR Assay (AMPLICOR CMV Test) versus pp65 antigenemia assay for monitoring cytomegalovirus viremia and guiding preemptive ganciclovir therapy in allogeneic stem cell transplantation. J Clin Microbiol 39:3938–3941
Ljungman P, Plotkin S (1995) Workshop on CMV disease—definitions, clinical severity scores and new syndromes. Scand J Infect Dis Suppl 99:87–89
Przepiorka D, Weisdorf D, Martin P et al (1994) Consensus conference on Acute GVHD grading. Bone Marrow Transplant 15:825–828
Jabs DA, Forman M, Enger C et al (1999) Comparison of cytomegalovirus loads in plasma and leukocytes of patients with cytomegalovirus retinitis. J Clin Microbiol 37:1431–1435
Pellegrin I, Garrigue I, Ekouevi D et al (2000) New molecular assays to predict occurrence of cytomegalovirus disease in renal transplant recipients. J Infect Dis 182:36–42
Schafer P, Tenschert W, Cremaschi L, et al (2001) Area under the viraemia curve versus absolute viral load: utility for predicting symptomatic cytomegalovirus infections in kidney transplant patients. J Med Virol 65:85–89
Boeckh M, Hawkins G, Myerson D et al (1997) Plasma PCR for cytomegalovirus DNA after allogeneic marrow transplantation: comparison with PCR using peripheral blood leukocytes, pp65 antigenemia and viral culture. Transplantation 64:108–113
Ksouri H, Eljed H, Greco A et al (2007) Analysis of cytomegalovirus (CMV) viremia using the pp65 antigenemia assay, the amplicor CMV test, and a semi- quantitative polymerase chain reaction test after allogenic marrow transplantation. Transpl Infect Dis 9:16–21
Garrigue I, Boucher S, Couzi L et al (2006) Whole blood real-time quantitative PCR for cytomegalovirus infection follow-up in transplant recipients. J Clin Virol 36:72–75
Mhiri L, Kaabi B, Houimel M, Arrouji Z, Slim A (2007) Comparison of pp65 antigenemia, quantitative PCR and DNA hybrid capture for detection of cytomegalovirus in transplant recipients and AIDS patients. J Virol Methods 143:23–28
Schvoerer E, Henriot S, Zachary P et al (2005) Monitoring low cytomegalovirus viremia in transplanted patients by a real-time PCR on plasma. J Med Virol 76:76–81
Martin-Davila P, Fortun J, Gutierrez C et al (2005) Analysis of a quantitative PCR assay for CMV infection in liver transplant recipients: an intent to find the optimal cut-off value. J Clin Virol 33:138–144
Gouarin S, Vabret A, Gault E et al (2004) Quantitative analysis of HCMV DNA load in whole blood of renal transplant patients using real-time PCR assay. J Clin Virol 29:194–201
Li H, Dummer S, Estes WR, et al (2003) Measurement of human cytomegalovirus loads by quantitative real-time PCR for monitoring clinical intervention in transplant recipients. J Clin Microbiol 41:187–191
Mengelle C, Sandres-Saun’e K, Pasquier C et al (2003) Automated extraction and quantification of human cytomegalovirus DNA in whole blood by real-time PCR assay. J Clin Microbiol 41:3840–3845
Guiver M, Fox AJ, Mutton K, Mogulkoc N, Egan J (2001) Evaluation of CMV viral load using TaqMan CMV quantitative PCR and comparison with CMV antigenemia in heart and lung transplant recipients. Transplant 71:1609–1615
Drew WL (2007) Laboratory diagnosis of cytomegalovirus infection and disease in immunocompromised patients. Curr Opin Infect Dis 20:408–411
Lengerke C, Ljubicic T, Meisner C et al (2006) Evaluation of the COBAS Amplicor HCMV Monitor for early detection and monitoring of human cytomegalovirus infection after allogenic stem cell transplantation. Bone Marrow Transplant 38:53–60
Allice T, Enrietto M, Pittaluga F et al (2006) Quantitation of cytomegalovirus DNA by real-time polymerase chain reaction in peripheral blood specimens of patients with solid organ transplants: comparison with end-point PCR and pp65 antigen test. J Med Virol 78:915–922
Pumannova M, Roubalova K, Vitek A, Sajdova J (2006) Comparison of quantitative competitive polymerase chain reaction-enzyme-linked immunosorbent assay with LightCycler-based polymerase chain reaction for measuring cytomegalovirus DNA in patients after hematopoietic stem cell transplantation. Diagn Microbiol Infect Dis 54:115–120
Mori T, Mori S, Kanda Y et al (2004) Clinical significance of cytomegalovirus (CMV) antigenemia in the prediction and diagnosis of CMV gastrointestinal disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 33:431–434
Weinberg A, Hodge TN, Li S et al (2000) Comparison of PCR, antigenemia assay, and rapid blood culture for detection and prevention of cytomegalovirus disease after lung transplantation. J Clin Microbiol 38(2):768–772
Ikewaki J, Ohtsuka E, Kawano R et al (2003) Real-time PCR assay compared to nested PCR and antigenemia assays for detecting cytomegalovirus reactivation in adult T-cell leukemia-lymphoma patients. J Clin Microbiol 41:4382–4387
Acknowledgement
This work was supported by grants from Hematology, Oncology and BMT Research Center, Tehran University Medical Sciences. We would like to thank Mr. Chahardouli for assistance in lab and Dr. Shamshiri for assistance in the statistical analysis. We would like to thank all clinicians and nurses who participated and provided material for this study. In particular we acknowledge Drs. Jahani, Mosavi, Bibordi, Bahar, Irvani.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghaffari, S.H., Obeidi, N., Dehghan, M. et al. Monitoring of Cytomegalovirus Reactivation in Bone Marrow Transplant Recipients by Real-time PCR. Pathol. Oncol. Res. 14, 399–409 (2008). https://doi.org/10.1007/s12253-008-9030-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-008-9030-3