Abstract
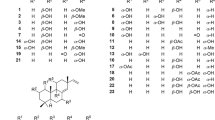
Despite the success of antiretroviral therapy (ART), efforts to develop new classes of antiviral agents have been hampered by the emergence of drug resistance. Dibenzo-indole-bearing aristolactams are compounds that have been isolated from various plants species and which show several clinically relevant effects, including anti-inflammatory, antiplatelet, and anti-mycobacterial actions. However, the effect of these compounds on human immunodeficiency virus type 1 (HIV-1) infection has not yet been studied. In this study, we discovered an aristolactam derivative bearing dibenzo[cd,f]indol-4(5H)-one that had a potent anti-HIV-1 effect. A structure-activity relationship (SAR) study using nine synthetic derivatives of aristolactam identified the differing effects of residue substitutions on the inhibition of HIV-1 infection and cell viability. Among the compounds tested, 1,2,8,9-tetramethoxy-5-(2-(piperidin-1-yl)ethyl)-dibenzo[cd,f]indol-4(5H)-one (Compound 2) exhibited the most potent activity by inhibiting HIV-1 infection with a half-maximal inhibitory concentration (IC50) of 1.03 μmol/L and a half-maximal cytotoxic concentration (CC50) of 16.91 μmol/L (selectivity index, 16.45). The inhibitory effect of the compounds on HIV-1 infection was linked to inhibition of the viral replication cycle. Mode-of-action studies showed that the aristolactam derivatives did not affect reverse transcription or integration; instead, they specifically inhibited Tat-mediated viral transcription. Taken together, these findings show that several aristolactam derivatives impaired HIV-1 infection by inhibiting the activity of Tat-mediated viral transcription, and suggest that these derivatives could be antiviral drug candidates.




Similar content being viewed by others
References
Agbottah E, de La Fuente C, Nekhai S, Barnett A, Gianella-Borradori A, Pumfery A, Kashanchi F (2005) Antiviral activity of CYC202 in HIV-1-infected cells. J Biol Chem 280:3029–3042
Arts EJ, Hazuda DJ (2012) HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med 2:a007161
Bedini E, Carabellese A, Barone G, Parrilli M (2005) First synthesis of the beta-D-rhamnosylated trisaccharide repeating unit of the O-antigen from Xanthomonas campestris pv. campestris 8004. J Org Chem 70:8064–8070
Cary DC, Peterlin BM (2018) Natural products and HIV/AIDS. AIDS Res Hum Retroviruses 34:31–38
Chia YC, Chang FR, Teng CM, Wu YC (2000) Aristolactams and dioxoaporphines from Fissistigma balansae and Fissistigma oldhamii. J Nat Prod 63:1160–1163
Choi YL, Kim JK, Choi SU, Min YK, Bae MA, Kim BT, Heo JN (2009) Synthesis of aristolactam analogues and evaluation of their antitumor activity. Bioorg Med Chem Lett 19:3036–3040
Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R (2016) HIV reservoirs: what, where and how to target them. Nat Rev Microbiol 14:55–60
Clouser CL, Patterson SE, Mansky LM (2010) Exploiting drug repositioning for discovery of a novel HIV combination therapy. J Virol 84:9301–9309
Couture A, Deniau E, Grandclaudon P, Rybalko-Rosen H, Leonce S, Pfeiffer B, Renard P (2002) Synthesis and biological evaluation of aristolactams. Bioorg Med Chem Lett 12:3557–3559
De Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH (1997) Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem 243:518–526
Esposito F, Tintori C, Martini R, Christ F, Debyser Z, Ferrarese R, Cabiddu G, Corona A, Ceresola ER, Calcaterra A, Iovine V, Botta B, Clementi M, Canducci F, Botta M, Tramontano E (2015) Kuwanon-L as a new allosteric HIV-1 integrase inhibitor: molecular modeling and biological evaluation. ChemBioChem 16:2507–2512
Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF (1997) Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300
Gibson KM, Steiner MC, Kassaye S, Maldarelli F, Grossman Z, Perez-Losada M, Crandall KA (2019) Corrigendum: a 28-year history of HIV-1 drug resistance and transmission in Washington. DC Front Microbiol 10:2590
Havlicek L, Hanus J, Vesely J, Leclerc S, Meijer L, Shaw G, Strnad M (1997) Cytokinin-derived cyclin-dependent kinase inhibitors: synthesis and cdc2 inhibitory activity of olomoucine and related compounds. J Med Chem 40:408–412
Hoarau C, Couture A, Cornet H, Deniau E, Grandclaudon P (2001) A concise total synthesis of the azaphenanthrene alkaloid eupolauramine. J Org Chem 66:8064–8069
Hsu MC, Schutt AD, Holly M, Slice LW, Sherman MI, Richman DD, Potash MJ, Volsky DJ (1991) Inhibition of HIV replication in acute and chronic infections in vitro by a Tat antagonist. Science 254:1799–1802
Ivanov A, Lin X, Ammosova T, Ilatovskiy AV, Kumari N, Lassiter H, Afangbedji N, Niu X, Petukhov MG, Nekhai S (2018) HIV-1 Tat phosphorylation on Ser-16 residue modulates HIV-1 transcription. Retrovirology 15:39
Jayasuriya H, Lingham RB, Graham P, Quamina D, Herranz L, Genilloud O, Gagliardi M, Danzeisen R, Tomassini JE, Zink DL, Guan Z, Singh SB (2002) Durhamycin A, a potent inhibitor of HIV Tat transactivation. J Nat Prod 65:1091–1095
Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH (1996) Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J Med Chem 39:1016–1017
Kashman Y, Gustafson KR, Fuller RW, Cardellina JH 2nd, McMahon JB, Currens MJ, Buckheit RW Jr, Hughes SH, Cragg GM, Boyd MR (1992) The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree. Calophyllum lanigerum. J Med Chem 35:2735–2743
Kim SR, Sung SH, Kang SY, Koo KA, Kim SH, Ma CJ, Lee HS, Park MJ, Kim YC (2004) Aristolactam BII of Saururus chinensis attenuates glutamate-induced neurotoxicity in rat cortical cultures probably by inhibiting nitric oxide production. Planta Med 70:391–396
Kumar V, Poonam Prasad AK, Parmar VS (2003) Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat Prod Rep 20:565–583
Margolis AM, Heverling H, Pham PA, Stolbach A (2014) A review of the toxicity of HIV medications. J Med Toxicol 10:26–39
Mitsuya H, Broder S (1987) Strategies for antiviral therapy in AIDS. Nature 325:773–778
Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC, Bolognesi D, Barry DW, Broder S (1985) 3’-Azido-3’-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci USA 82:7096–7100
Mousseau G, Valente S (2012) Strategies to block HIV transcription: focus on small molecule tat inhibitors biology (Basel) 1:668–697
Mousseau G, Clementz MA, Bakeman WN, Nagarsheth N, Cameron M, Shi J, Baran P, Fromentin R, Chomont N, Valente ST (2012) An analog of the natural steroidal alkaloid cortistatin a potently suppresses Tat-dependent HIV transcription. Cell Host Microbe 12:97–108
Pang R, Zhang C, Yuan D, Yang M (2008) Design and SAR of new substituted purines bearing aryl groups at N9 position as HIV-1 Tat-TAR interaction inhibitors. Bioorg Med Chem 16:8178–8186
Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D (1998) Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 72:2855–2864
Reddy MC, Jeganmohan M (2017) Total synthesis of aristolactam alkaloids via synergistic C–H bond activation and dehydro-Diels-Alder reactions. Chem Sci 8:4130–4135
Schang LM (2002) Cyclin-dependent kinases as cellular targets for antiviral drugs. J Antimicrob Chemother 50:779–792
Shin Y, Choi BS, Kim KC, Kang C, Kim K, Yoon CH (2017) Development of a dual reporter screening assay for distinguishing the inhibition of HIV Tat-mediated transcription from off-target effects. J Virol Methods 249:1–9
Shin Y, Kim HG, Park CM, Choi MS, Kim DE, Choi BS, Kim K, Yoon CH (2020) Identification of novel compounds against Tat-mediated human immunodeficiency virus-1 transcription by high-throughput functional screening assay. Biochem Biophys Res Commun 523:368–374
Tsai IL, Lee FP, Wu CC, Duh CY, Ishikawa T, Chen JJ, Chen YC, Seki H, Chen IS (2005) New cytotoxic cyclobutanoid amides, a new furanoid lignan and anti-platelet aggregation constituents from Piper arborescens. Planta Med 71:535–542
Wei P, Garber ME, Fang SM, Fischer WH, Jones KA (1998) A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451–462
Xu H, Lv M (2009) Developments of indoles as anti-HIV-1 inhibitors. Curr Pharm Des 15:2120–2148
Yao T, Larock RC (2005) Regio- and stereoselective synthesis of isoindolin-1-ones via electrophilic cyclization. J Org Chem 70:1432–1437
Yoon CH, Kim SY, Byeon SE, Jeong Y, Lee J, Kim KP, Park J, Bae YS (2015) p53-derived host restriction of HIV-1 replication by protein kinase R-mediated Tat phosphorylation and inactivation. J Virol 89:4262–4280
Yu X, Lin W, Li J, Yang M (2004) Synthesis and biological evaluation of novel beta-carboline derivatives as Tat-TAR interaction inhibitors. Bioorg Med Chem Lett 14:3127–3130
Yuan D, He M, Pang R, Lin SS, Li Z, Yang M (2007) The design, synthesis, and biological evaluation of novel substituted purines as HIV-1 Tat-TAR inhibitors. Bioorg Med Chem 15:265–272
Zhang YN, Zhong XG, Zheng ZP, Hu XD, Zuo JP, Hu LH (2007) Discovery and synthesis of new immunosuppressive alkaloids from the stem of Fissistigma oldhamii (Hemsl.) Merr. Bioorg Med Chem 15:988–996
Zhang HJ, Rumschlag-Booms E, Guan YF, Wang DY, Liu KL, Li WF, Nguyen VH, Cuong NM, Soejarto DD, Fong HHS, Rong L (2017) Potent inhibitor of drug-resistant HIV-1 strains identified from the medicinal plant justicia gendarussa. J Nat Prod 80:1798–1807
Acknowledgements
This work was supported by grants from the Korea National Institute of Health (Grant Number: 2019-NI-066-00 and 2020-ER5106-00).
Author information
Authors and Affiliations
Contributions
CHY conceived the project. YS and CMP mainly conducted the experiments and participated in the drafting of the manuscript. HGK, MSC, DEK, BSC, KK discussed and analyzed the data. CHY wrote the manuscript. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shin, Y., Park, C.M., Kim, H.G. et al. Identification of Aristolactam Derivatives That Act as Inhibitors of Human Immunodeficiency Virus Type 1 Infection and Replication by Targeting Tat-Mediated Viral Transcription. Virol. Sin. 36, 254–263 (2021). https://doi.org/10.1007/s12250-020-00274-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-020-00274-7