Abstract
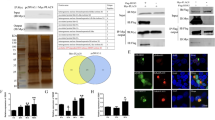
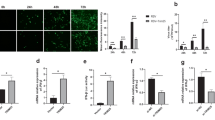
MicroRNAs (miRNAs) encoded by latency-associated transcript are associated with both latent and acute stages of herpes simplex virus 2 (HSV-2) infection. In this study, miRNA-H4-5p and miRNA-H4-3p were ectopically expressed in HeLa cells to explore potential cellular targets of viral miRNAs and demonstrate their potential biological functions. The results showed that miRNA-H4-5p could reverse apoptosis induced by actinomycin D (Act-D) and promote cell cycle progression, but miRNA-H4-3p had no such obvious functions. Bioinformatics analysis, luciferase report assay, quantitative reverse transcription polymerase chain reaction (qRT-PCR), and Western blotting demonstrated that miRNA-H4-5p could bind to the 3′-untranslated region (UTR) of cyclin-dependent kinase inhibitor 2A (CDKN2A) and cyclin-dependent kinase-like 2 (CDKL2) to negatively regulate their expression. We verified that these two targeted genes were associated with cell apoptosis and cell cycle. Furthermore, in HeLa cells infected with HSV-2, we detected significantly reduced expression of CDKN2A and CDKL2 and demonstrated the negative regulation effect of miRNA-H4-5p on these two target genes. Our findings show that viral miRNAs play a vital role in regulating the expression of the host’s cellular genes that participate in cell apoptosis and progression to reshape the cellular environment in response to HSV-2 infection, providing further information on the roles of encoded herpesvirus miRNAs in pathogen–host interaction.





Similar content being viewed by others
References
Afonso-Grunz F, Müller S (2015) Principles of miRNA–mRNA interactions: beyond sequence complementarity. Cell Mol Life Sci 72:3127–3141
Bartel DP (2009) microRNAs: target recognition and regulatory functions. Cell 136:215–233
Cullen BR (2009) Viral and cellular messenger RNA targets of viral microRNAs. Nature 457:421–425
Cullen BR (2011) Herpesvirus microRNAs: phenotypes and functions. Curr Opin Virol 1:211–215
Dalmay T (2013) Mechanism of miRNA-mediated repression of mRNA translation. Essays Biochem 54:29–38
Du T, Han Z, Zhou G, Roizman B (2015) Patterns of accumulation of miRNAs encoded by herpes simplex virus during productive infection, latency, and on reactivation. Proc Natl Acad Sci USA 112:E49–E55
Duan F, Liao J, Huang Q, Nie Y, Wu K (2012) HSV-1 miR-H6 inhibits HSV-1 replication and IL-6 expression in human corneal epithelial cells in vitro. Clin Dev Immunol 2012:192791
Everett RD (2000) ICP0, a regulator of herpes simplex virus during lytic and latent infection. BioEssays 22:761–770
Goldberg IH, Rabinowitz M (1962) Actionmycin D inhibition of deoxyribonucleic acid-dependent synthesis of ribonucleic acid. Science 136:315–316
Gomi H, Sassa T, Thompson RF, Itohara S (2010) Involvement of cyclin-dependent kinase-like 2 in cognitive function required for contextual and spatial learning in mice. Front Behav Neurosci 4:17
Gottwein E, Cullen BR (2008) Viral and cellular microRNAs as determinants in pathogenesis and immunity. Cell Host Microbe 3:375–387
Grey F (2015) Role of microRNAs in herpesvirus latency and persistence. J Gen Virol 96:739–751
Gupta A, Gartner JJ, Sethupathy P, Hatzigeorgiou AG, Fraser NW (2006) Anti-apoptotic function of a microRNA encoded by the HSV-1 latency-associated transcript. Nature 442:82–85
Hooykaas MJ, Kruse E, Wiertz EJ, Lebbink RJ (2016) Comprehensive profiling of functional Epstein–Barr virus miRNA expression in human cell lines. BMC Genom 17:644
Journey LJ, Goldstein MN (1961) Electron microscope studies on HeLa cell lines sensitive and resistant to actinomycin D. Cancer Res 21:929–932
Jurak I, Kramer MF, Mellor JC, van Lint AL, Roth FP, Knipe DM, Coen DM (2010) Numerous conserved and divergent microRNAs expressed by herpes simplex viruses 1 and 2. J Virol 84:4659–4672
Kleeff J, Kornmann M, Sawhney H, Korc M (2000) Actinomycin D induces apoptosis and inhibits growth of pancreatic cancer cells. Int J Cancer 86:399–407
Li L, Liu C, Amato RJ, Chang JT, Du G, Li W (2014) CDKL2 promotes epithelial-mesenchymal transition and breast cancer progression. Oncotarget 5:10840–10853
Liu Y, Yang HL, Zhong FF, Fan JY (2016) Anti-apoptotic function of herpes simplex virus-2 latency-associated transcript RL1 sequence and screening of its encoded microRNAs. Clin Exp Dermatol 41:782–791
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Looker KJ, Elmes JAR, Gottlieb SL, Schiffer JT, Vickerman P, Turner KME, Boily MC (2017a) Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect 17:1303–1316
Looker KJ, Magaret AS, May MT, Turner KME, Vickerman P, Newman LM, Gottlieb SL (2017b) First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health 5:e300–e309
Lu DF, Wang YS, Li C, Wei GJ, Chen R, Dong DM, Yao M (2015) Actinomycin D inhibits cell proliferations and promotes apoptosis in osteosarcoma cells. Int J Clin Exp Med 8:1904–1911
Niederacher D, Yan HY, An HX, Bender HG, Beckmann MW (1999) CDKN2A gene inactivation in epithelial sporadic ovarian cancer. Br J Cancer 80:1920–1926
Pal A, Potjer TP, Thomsen SK, Ng HJ, Barrett A, Scharfmann R, James TJ, Bishop DT, Karpe F, Godsland IF, Vasen HF, Newton-Bishop J, Pijl H, McCarthy MI, Gloyn AL (2016) Loss-of-function mutations in the cell-cycle control gene CDKN2A impact on glucose homeostasis in humans. Diabetes 65:527–533
Pfeffer S, Zavolan M, Grässer FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T (2004) Identification of virus-encoded microRNAs. Science 304:734–736
Piedade D, Azevedo-Pereira JM (2016) The role of microRNAs in the pathogenesis of herpesvirus infection. Viruses 8:E156
Salic A, Mitchison TJ (2008) A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA 105:2415–2420
Skalsky RL, Cullen BR (2010) Viruses, microRNAs, and host interactions. Annu Rev Microbiol 64:123–141
Tang S, Bertke AS, Patel A, Wang K, Cohen JI, Krause PR (2008) An acutely and latently expressed herpes simplex virus 2 viral microRNA inhibits expression of ICP34.5, a viral neurovirulence factor. Proc Natl Acad Sci USA 105:10931–10936
Tang S, Patel A, Krause PR (2009) Novel less-abundant viral microRNAs encoded by herpes simplex virus 2 latency-associated transcript and their roles in regulating ICP34.5 and ICP0 mRNAs. J Virol 83:1433–1442
Tang S, Bertke AS, Patel A, Margolis TP, Krause PR (2011) Herpes simplex virus 2 microRNA miR-H6 is a novel latency-associated transcript-associated microRNA, but reduction of its expression does not influence the establishment of viral latency or the recurrence phenotype. J Virol 85:4501–4509
Tang S, Bosch-Marce M, Patel A, Margolis TP, Krause PR (2015) Characterization of herpes simplex virus 2 primary microRNA transcript regulation. J Virol 89:4837–4848
Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR (2008) microRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 454:780–783
Umbach JL, Wang K, Tang S, Krause PR, Mont EK, Cohen JI, Cullen BR (2010) Identification of viral microRNAs expressed in human sacral ganglia latently infected with herpes simplex virus 2. J Virol 84:1189–1192
Whitley RJ (2015) Herpes simplex virus infections of the central nervous system. Continuum (Minneap Minn) 21:1704–1713
Wu W, Guo Z, Zhang X, Guo L, Liu L, Liao Y, Wang J, Wang L, Li Q (2013) A microRNA encoded by HSV-1 inhibits a cellular transcriptional repressor of viral immediate early and early genes. Sci China Life Sci 56:373–383
Yamada M, Banno Y, Takuwa Y, Koda M, Hara A, Nozawa Y (2004) Overexpression of phospholipase D prevents actinomycin D-induced apoptosis through potentiation of phosphoinositide 3-kinase signalling pathways in Chinese-hamster ovary cells. Biochem J 378:649–656
Zheng SQ, Li YX, Zhang Y, Li X, Tang H (2011) miR-101 regulates HSV-1 replication by targeting ATP5B. Antivir Res 89:219–226
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81371749) and (81171511).
Author information
Authors and Affiliations
Contributions
HY and JF designed the study; YZ, JY and YL performed the experiments; YZ and JY analyzed the data; YZ and YL wrote the manuscript; HY and JF finalized the manuscript. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
The authors declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Yang, J., Liu, Y. et al. HSV-2-encoded miRNA-H4 Regulates Cell Cycle Progression and Act-D-induced Apoptosis in HeLa Cells by Targeting CDKL2 and CDKN2A. Virol. Sin. 34, 278–286 (2019). https://doi.org/10.1007/s12250-019-00101-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-019-00101-8